S
Slownickel
- 78
- 33
SlowNickel does Gypsum effect soil ph ????
Cause i am understanding it does not.
From your data it does! We have seen this before, especially when the soil is loaded with carbonates.
Everything depends where you are standing. I have worked in more than 24 countries at last count. We aren't in Kansas anymore as Dorothy said. AGVISE is in North Dakota and Minnesota. They say that there are no carbonates in a pH of less than 7.4 I wish that were the case. But that is because I am in a low rainfall area, the coast of Peru. I am not in North Dakota. If we don't apply the right soil analysis procedures, we can often get the incorrect answer due to bad analysis. I propose this is the case.AGVISE recently conducted a laboratory project
The dosis of 36000 lb/a of gyp is a bit absurd. Let's go up and look at reasonable or slightly high rates. There we have a pH of 7.3 You know what that means? That means we have 2.5 times more acidity than before! So the pH did drop. That would be statistically dramatic if we ran some statistics.It is apparent that this is not true, even at rates as high as 36000 lb/a gypsum the soil pH is about the same as the check.
As the rate of gypsum is increased you can see the test level for calcium increases while the level of magnesium, potassium and sodium remain the same (Figure 2).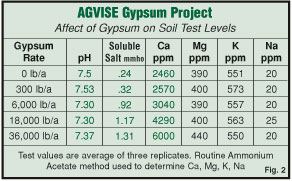
This is not correct. We have now changed the chemistry dramatically. You are stuck on ppms. It is not about ppms. It is about relationships at the Meq level, electrical charges and the balancing there of.
When gypsum (calcium sulfate) is applied to the soil, it dissolves in the soil solution. Some of the calcium becomes attached to soil particles as part of the cation exchange capacity of the soil. The remaining gypsum stays in the soil solution as dissolved calcium sulfate salt. The soil testing method used by all commercial and University soil testing laboratories picks up the calcium that is held on the soil, as well as the calcium that is dissolved in the soil solution as soluble salts. The calcium in the soil solution is “NOT” held on the soil, and should not be included because it is not held on the soil, but it is included in the common method used by all soil testing laboratories. Because this soil testing method includes the calcium from the soil solution, the test values reported are inflated on the high side. You can see this inflation occurring as the rate of gypsum increases in Figure 2. The calcium test value goes up, but the soil is not holding more calcium, the test is just including the calcium dissolved in the salts in the soil solution. You can see this is true because the salt level increases as the rate of gypsum increases.
The base saturation value for a soil is a calculation that determines the percent each cation makes up of the total cations in the soil. When the percent base saturation for a soil is calculated, the ppm value for calcium, magnesium, potassium and sodium are used in the calculation. Since the calcium ppm level keeps increasing as more gypsum is applied, calcium becomes a larger percentage of the total cations. We know that the soil is not holding more calcium, we are just measuring the increasing amount of calcium in the salts of the soil solution.
The Cation Exchange Capacity (CEC) of a soil is the ability of a soil to hold the cations calcium, magnesium, potassium and sodium. The CEC of a soil is a permanent feature based primarily on soil texture, clay content and organic matter. When gypsum is applied to the soil it does not actually change the CEC of the soil, but it does change the calcium test value determined in the laboratory, which is used to calculate the CEC value for the soil. Because an inflated calcium value is used to calculate the CEC of the soil, the calculated CEC goes up as the gypsum rate increases (Figure 3).These CEC values are erroneous due to the error caused by including calcium from the salts in the soil solution. The correct CEC of this soil, determined by a special laboratory method that does not include calcium from the salts in the soil solution is 18 meq. The routine method, used by all commercial soil testing labs, did a good job of determining the CEC to be 17, until higher rates of gypsum were applied. That means that you can change the calculated CEC of the soil by adding gypsum, but you are not really changing the ability of the soil to hold cations.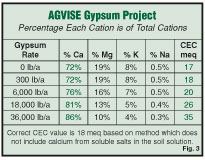
Erroneous because the lab used the wrong procedures. And as you can clearly see those gypsum applications have have moved the soil solution to where it needed to go, in fact, went a tad to much with the 18 tons/acre.
Facts learned from past field research and AGVISE laboratory project:
- You can achieve high yields on soils with a wide range of cation ratios.
1. You can apply enough gypsum to a soil to change the laboratory test results for calcium, but this does not actually change the CEC of the soil or the amount of each cations actually held on the soil.
I disagree, in the case you start breaking down the carbonates in the soil, this generates food for micros (carbon) from the carbonates and at the same time the distribution of bases are swayed, which is the idea! Change the relationships!
This why we add gypsum to flocculate the soil and open it up!2. Even low rates of gypsum over the long term on a poorly drained soil will increase the salt level of the soil. As the soil salt level increases, crop yields will decrease over time.
Please read here... the Univ of Arizona disagrees with this lab from North Dakota and Minnesota. http://extension.arizona.edu/sites/extension.arizona.edu/files/pubs/az1413.pdf
The data disagrees with you, each point of pH is 10 x more acid or alkalinity (pH is exponential). Gypsum acidified this soil 2.5 times. Excellent data!4. Gypsum does not decrease or increase the soil pH of productive soils
AGREED. Minimum levels are critical and are another conversation. Looking forward to that one!5. It is most important to know the level of each nutrient in the soil. If a nutrient tests in the deficient range, it needs to be applied. ]
Might I suggest some free reading material that might convince you otherwise? Soilandhealth dot org library. Victor Tiedjens, "More Food from Soil Science" .The concept of balancing cations is not supported by the facts of the real world.
Remember the number 1 killer for plants is salts :) have a nice day folks think of it as a magic amendment ,, if you like if you think its doing you better then by all mean use it at the end of the day appears that you can grow nice healthy plants with out it,, like mine :) fist picture salt buiild up its inevitable with gypsuim Cause god forbid sulfate is not a salt
I suggest you read up on the SAR or sodium absorbtion ratios. Those same concepts pertain to soils.
The works above that you quoted help prove my point. The base distribution did move, it lowered Mg, lowered both K it lowerd Na and it increased Ca, exactly what I do!!!
The variation in dosis was humorous. I pull up around 6,000 to 8,000 lbs to the acre.
Note in this case, the gypsum not only helped bring up the base distributions but it also lowered the pH, again, in a soil of a pH 7.5 to 7.3 or 2 times more acidity! That is impressive, could you please send me the exact url where you got this data to chase down the original work? I could use the data for the book I am writing.
There was also a very large error in the methodology used in this soil analysis. Undoubtedly this was ammonium acetate at a pH of 7. At a pH of 7, the Ca quantities are grossly overstated. https://goo.gl/tu1id6 for your reading pleasure, thanks to the PGA.
As you will see, the correct method to remove the calcium carbonate influence is to you [email protected]
Also there was no salinity in this example, terribly low conductivity and NA at 20 ppm.
I don't see they grew any crops in what you showed us.
I am not here to argue, I am trying to bring real science, not the science pushed by the Potassium and Phosphorus Institute that tried very hard to get rid of the importance of calcium balances.
One last point, not only does the idea of balancing the bases make sense, I will scare the hell out of you when we start talking about iron and manganese relationships.
Realize this is what I do for a living. Not a plant in my backyard. I have worked in nutrition for several multinationals and always championed the numbers.
My grow https://goo.gl/1U92jS none of these trees are more than 2.5 years old. Many have only 6 months or so in these photos... Note the dark salts in between the rows on some of the citrus photos.
I uploaded a file for you to look at. Take a good hard look at the Ca/Mg relationships, most especially sample 2. Grab the THC levels from table 6 and pencil them into Table 3, next to the foliar calcium levels....
Imagine... upto 600% more THC in the sample with the MOST Ca and nearly the lowest Mg!!!
Good day my friend! Got me motivated, love a good conversation!
Last edited:





