Bill Murry
- 337
- 93
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3165946/
Abstract
Tetrahydrocannabinol (THC) has been the primary focus of cannabis research since 1964, when Raphael Mechoulam isolated and synthesized it. More recently, the synergistic contributions of cannabidiol to cannabis pharmacology and analgesia have been scientifically demonstrated. Other phytocannabinoids, including tetrahydrocannabivarin, cannabigerol and cannabichromene, exert additional effects of therapeutic interest. Innovative conventional plant breeding has yielded cannabis chemotypes expressing high titres of each component for future study. This review will explore another echelon of phytotherapeutic agents, the cannabis terpenoids: limonene, myrcene, α-pinene, linalool, β-caryophyllene, caryophyllene oxide, nerolidol and phytol. Terpenoids share a precursor with phytocannabinoids, and are all flavour and fragrance components common to human diets that have been designated Generally Recognized as Safe by the US Food and Drug Administration and other regulatory agencies. Terpenoids are quite potent, and affect animal and even human behaviour when inhaled from ambient air at serum levels in the single digits ng·mL−1. They display unique therapeutic effects that may contribute meaningfully to the entourage effects of cannabis-based medicinal extracts. Particular focus will be placed on phytocannabinoid-terpenoid interactions that could produce synergy with respect to treatment of pain, inflammation, depression, anxiety, addiction, epilepsy, cancer, fungal and bacterial infections (including methicillin-resistant Staphylococcus aureus). Scientific evidence is presented for non-cannabinoid plant components as putative antidotes to intoxicating effects of THC that could increase its therapeutic index. Methods for investigating entourage effects in future experiments will be proposed. Phytocannabinoid-terpenoid synergy, if proven, increases the likelihood that an extensive pipeline of new therapeutic products is possible from this venerable plant.
LINKED ARTICLES
This article is part of a themed issue on Cannabinoids in Biology and Medicine. To view the other articles in this issue visit
Keywords: cannabinoids, terpenoids, essential oils, THC, CBD, limonene, pinene, linalool, caryophyllene, phytotherapy
Go to:
The roots of cannabis synergy
Cannabis has been a medicinal plant of unparalleled versatility for millennia (Mechoulam, 1986; Russo, 2007; 2008;), but whose mechanisms of action were an unsolved mystery until the discovery of tetrahydrocannabinol (THC) (Gaoni and Mechoulam, 1964a), the first cannabinoid receptor, CB1 (Devane et al., 1988), and the endocannabinoids, anandamide (arachidonoylethanolamide, AEA) (Devane et al., 1992) and 2-arachidonoylglycerol (2-AG) (Mechoulam et al., 1995; Sugiura et al., 1995). While a host of phytocannabinoids were discovered in the 1960s: cannabidiol (CBD) (Mechoulam and Shvo, 1963), cannabigerol (CBG) (Gaoni and Mechoulam, 1964b), cannabichromene (CBC) (Gaoni and Mechoulam, 1966), cannabidivarin (CBDV) (Vollner et al., 1969) and tetrahydrocannabivarin (THCV) (Gill et al., 1970), the overwhelming preponderance of research focused on psychoactive THC. Only recently has renewed interest been manifest in THC analogues, while other key components of the activity of cannabis and its extracts, the cannabis terpenoids, remain understudied (McPartland and Russo, 2001b; Russo and McPartland, 2003). The current review will reconsider essential oil (EO) agents, their peculiar pharmacology and possible therapeutic interactions with phytocannabinoids. Nomenclature follows conventions inAlexander et al. (2009).
Phytocannabinoids and terpenoids are synthesized in cannabis, in secretory cells inside glandular trichomes (Figure 1) that are most highly concentrated in unfertilized female flowers prior to senescence (Potter, 2004;Potter, 2009). Geranyl pyrophosphate is formed as a precursor via the deoxyxylulose pathway in cannabis (Fellermeier et al., 2001), and is a parent compound to both phytocannabinoids and terpenoids (Figure 2). After coupling with either olivetolic acid or divarinic acid, pentyl or propyl cannabinoid acids are produced, respectively, via enzymes that accept either substrate (de Meijer et al., 2003), a manifestation of Mechoulam's postulated ‘Nature's Law of Stinginess’. Although having important biochemical properties in their own right, acid forms of phytocannabinoids are most commonly decarboxylated via heat to produce the more familiar neutral phytocannabinoids (Table 1). Alternatively, geranyl pyrophosphate may form limonene and other monoterpenoids in secretory cell plastids, or couple with isopentenyl pyrophosphate in the cytoplasm to form farnesyl pyrophosphate, parent compound to the sesquiterpenoids, that co-localizes with transient receptor potential vanilloid receptor (TRPV) 1 in human dorsal root ganglion, suggesting a role in sensory processing of noxious stimuli (Bradshaw et al., 2009), and which is the most potent endogenous ligand to date on the G-protein coupled receptor (GPR) 92 (Oh et al., 2008).
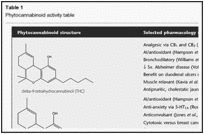
Table 1
Phytocannabinoid activity table

Figure 1
Cannabis capitate glandular (EBR by permission of Bedrocan BV, Netherlands).

Figure 2
Phytocannabinoid and cannabis terpenoid biosynthesis.
An obvious question pertains to the chemical ecology of such syntheses that require obvious metabolic demands on the plant (Gershenzon, 1994), and these will be considered.
Is cannabis merely a crude vehicle for delivery of THC? Might it rather display herbal synergy (Williamson, 2001) encompassing potentiation of activity by active or inactive components, antagonism (evidenced by the ability of CBD to reduce side effects of THC; Russo and Guy, 2006), summation, pharmacokinetic and metabolic interactions? Recently, four basic mechanisms of synergy have been proposed (Wagner and Ulrich-Merzenich, 2009): (i) multi-target effects; (ii) pharmacokinetic effects such as improved solubility or bioavailability; (iii) agent interactions affecting bacterial resistance; and (iv) modulation of adverse events. Cannabis was cited as an illustration.
Could phytocannabinoids function analogously to the endocannabinoid system (ECS) with its combination of active and ‘inactive’ synergists, first described as an entourage (Ben-Shabat et al., 1998), with subsequent refinement (Mechoulam and Ben-Shabat, 1999) and qualification (p. 136): ‘This type of synergism may play a role in the widely held (but not experimentally based) view that in some cases plants are better drugs than the natural products isolated from them’. Support derives from studies in which cannabis extracts demonstrated effects two to four times greater than THC (Carlini et al., 1974); unidentified THC antagonists and synergists were claimed (Fairbairn and Pickens, 1981), anticonvulsant activity was observed beyond the cannabinoid fraction (Wilkinson et al., 2003), and extracts of THC and CBD modulated effects in hippocampal neurones distinctly from pure compounds (Ryan et al., 2006). Older literature also presented refutations: no observed differences were noted by humans ingesting or smoking pure THC versus herbal cannabis (Wachtel et al., 2002); pure THC seemed to account for all tetrad-type effects in mice (Varvel et al., 2005); and smoked cannabis with varying CBD or CBC content failed to yield subjective differences combined with THC (Ilan et al., 2005). Explanations include that the cannabis employed by Wachtel yielded 2.11% THC, but with only 0.3% cannabinol (CBN) and 0.05% CBD (Russo and McPartland, 2003), and Ilan's admission that CBN and CBD content might be too low to modulate THC. Another factor is apparent in that terpenoid yields from vaporization of street cannabis were 4.3–8.5 times of those from US National Institute on Drug Abuse cannabis (Bloor et al., 2008). It is undisputed that the black market cannabis in the UK (Potter et al., 2008), Continental Europe (King et al., 2005) and the USA (Mehmedic et al., 2010) has become almost exclusively a high-THC preparation to the almost total exclusion of other phytocannabinoids. If – as many consumers and experts maintain (Clarke, 2010) – there are biochemical, pharmacological and phenomenological distinctions between available cannabis ‘strains’, such phenomena are most likely related to relative terpenoid contents and ratios. This treatise will assess additional evidence for putative synergistic phytocannabinoid-terpenoid effects exclusive of THC, to ascertain whether this botanical may fulfil its promise as, ‘a neglected pharmacological treasure trove’ (Mechoulam, 2005).
Go to:
Phytocannabinoids, beyond THC: a brief survey
Phytocannabinoids are exclusively produced in cannabis (vide infra for exception), but their evolutionary and ecological raisons d'être were obscure until recently. THC production is maximized with increased light energy (Potter, 2009). It has been known for some time that CBG and CBC are mildly antifungal (ElSohly et al., 1982), as are THC and CBD against a cannabis pathogen (McPartland, 1984). More pertinent, however, is the mechanical stickiness of the trichomes, capable of trapping insects with all six legs (Potter, 2009). Tetrahydrocannabinolic acid (THCA) and cannabichromenic acid (Morimoto et al., 2007), as well as cannabidiolic acid and cannabigerolic acid (CBGA; Shoyama et al., 2008) produce necrosis in plant cells. Normally, the cannabinoid acids are sequestered in trichomes away from the flower tissues. Any trichome breakage at senescence may contribute to natural pruning of lower fan leaves that otherwise utilize energy that the plant preferentially diverts to the flower, in continued efforts to affect fertilization, generally in vain when subject to human horticulture for pharmaceutical production. THCA and CBGA have also proven to be insecticidal in their own right (Sirikantaramas et al., 2005).
Over 100 phytocannabinoids have been identified (Brenneisen, 2007; Mehmedic et al., 2010), but many are artefacts of analysis or are produced in trace quantities that have not permitted thorough investigation. The pharmacology of the more accessible phytocannabinoids has received excellent recent reviews (Pertwee et al., 2007; Izzo et al., 2009; De Petrocellis and Di Marzo, 2010; De Petrocellis et al., 2011), and will be summarized here, with emphasis on activities with particular synergistic potential.
THC (Table 1) is the most common phytocannabinoid in cannabis drug chemotypes, and is produced in the plant via an allele co-dominant with CBD (de Meijer et al., 2003). THC is a partial agonist at CB1 and cannabinoid receptor 2 (CB2) analogous to AEA, and underlying many of its activities as a psychoactive agent, analgesic, muscle relaxant and antispasmodic (Pacher et al., 2006). Additionally, it is a bronchodilator (Williams et al., 1976), neuroprotective antioxidant (Hampson et al., 1998), antipruritic agent in cholestatic jaundice (Neff et al., 2002) and has 20 times the anti-inflammatory power of aspirin and twice that of hydrocortisone (Evans, 1991). THC is likely to avoid potential pitfalls of either COX-1 or COX-2 inhibition, as such activity is only noted at concentrations far above those attained therapeutically (Stott et al., 2005).
CBD is the most common phytocannabinoid in fibre (hemp) plants, and second most prevalent in some drug chemotypes. It has proven extremely versatile pharmacologically (Table 1) (Pertwee, 2004; Mechoulam et al., 2007), displaying the unusual ability to antagonize CB1 at a low nM level in the presence of THC, despite having little binding affinity (Thomas et al., 2007), and supporting its modulatory effect on THC-associated adverse events such as anxiety, tachycardia, hunger and sedation in rats and humans (Nicholson et al., 2004; Murillo-Rodriguez et al., 2006; Russo and Guy, 2006). CBD is an analgesic (Costa et al., 2007), is a neuroprotective antioxidant more potent than ascorbate or tocopherol (Hampson et al., 1998), without COX inhibition (Stott et al., 2005), acts as a TRPV1 agonist analogous to capsaicin but without noxious effect (Bisogno et al., 2001), while also inhibiting uptake of AEA and weakly inhibiting its hydrolysis. CBD is an antagonist on GPR55, and also on GPR18, possibly supporting a therapeutic role in disorders of cell migration, notably endometriosis (McHugh et al., 2010). CBD is anticonvulsant (Carlini and Cunha, 1981;Jones et al., 2010), anti-nausea (Parker et al., 2002), cytotoxic in breast cancer (Ligresti et al., 2006) and many other cell lines while being cyto-preservative for normal cells (Parolaro and Massi, 2008), antagonizes tumour necrosis factor-alpha (TNF-α) in a rodent model of rheumatoid arthritis (Malfait et al., 2000), enhances adenosine receptor A2A signalling via inhibition of an adenosine transporter (Carrier et al., 2006), and prevents prion accumulation and neuronal toxicity (Dirikoc et al., 2007). A CBD extract showed greater anti-hyperalgesia over pure compound in a rat model with decreased allodynia, improved thermal perception and nerve growth factor levels and decreased oxidative damage (Comelli et al., 2009). CBD also displayed powerful activity against methicillin-resistant Staphylococcus aureus (MRSA), with a minimum inhibitory concentration (MIC) of 0.5–2 µg·mL−1 (Appendino et al., 2008). In 2005, it was demonstrated that CBD has agonistic activity at 5-hydroxytryptamine (5-HT)1A at 16 µM (Russo et al., 2005), and that despite the high concentration, may underlie its anti-anxiety activity (Resstel et al., 2009; Soares Vde et al., 2010), reduction of stroke risk (Mishima et al., 2005), anti-nausea effects (Rock et al., 2009) and ability to affect improvement in cognition in a mouse model of hepatic encephalopathy (Magen et al., 2009). A recent study has demonstrated that CBD 30 mg·kg−1 i.p. reduced immobility time in the forced swim test compared to imipramine (P < 0.01), an effect blocked by pre-treatment with the 5-HT1A antagonist WAY100635 (Zanelati et al., 2010), supporting a prospective role for CBD as an antidepressant. CBD also inhibits synthesis of lipids in sebocytes, and produces apoptosis at higher doses in a model of acne (vide infra). One example of CBD antagonism to THC would be the recent observation of lymphopenia in rats (CBD 5 mg·kg−1) mediated by possible CB2 inverse agonism (Ignatowska-Jankowska et al., 2009), an effect not reported in humans even at doses of pure CBD up to 800 mg (Crippa et al., 2010), possibly due to marked interspecies differences in CB2 sequences and signal transduction. CBD proved to be a critical factor in the ability of nabiximols oromucosal extract in successfully treating intractable cancer pain patients unresponsive to opioids (30% reduction in pain from baseline), as a high-THC extract devoid of CBD failed to distinguish from placebo (Johnson et al., 2010). This may represent true synergy if the THC–CBD combination were shown to provide a larger effect than a summation of those from the compounds separately (Berenbaum, 1989).
CBC (Table 1) was inactive on adenylate cyclase inhibition (Howlett, 1987), but showed activity in the mouse cannabinoid tetrad, but only at 100 mg·kg−1, and at a fraction of THC activity, via a non-CB1, non-CB2 mechanism (Delong et al., 2010). More pertinent are anti-inflammatory (Wirth et al., 1980) and analgesic activity (Davis and Hatoum, 1983), its ability to reduce THC intoxication in mice (Hatoum et al., 1981), antibiotic and antifungal effects (ElSohly et al., 1982), and observed cytotoxicity in cancer cell lines (Ligresti et al., 2006). A CBC-extract displayed pronounced antidepressant effect in rodent models (Deyo and Musty, 2003). Additionally, CBC was comparable to mustard oil in stimulating TRPA1-mediated Ca++in human embryonic kidney 293 cells (50–60 nM) (De Petrocellis et al., 2008). CBC recently proved to be a strong AEA uptake inhibitor (De Petrocellis et al., 2011). CBC production is normally maximal, earlier in the plant's life cycle (de Meijer et al., 2009a). An innovative technique employing cold water extraction of immature leaf matter from selectively bred cannabis chemotypes yields a high-CBC ‘enriched trichome preparation’ (Potter, 2009).
CBG (Table 1), the parent phytocannabinoid compound, has a relatively weak partial agonistic effect at CB1(Ki 440 nM) and CB2 (Ki 337 nM) (Gauson et al., 2007). Older work supports gamma aminobutyric acid (GABA) uptake inhibition greater than THC or CBD (Banerjee et al., 1975) that could suggest muscle relaxant properties. Analgesic and anti-erythemic effects and the ability to block lipooxygenase were said to surpass those of THC (Evans, 1991). CBG demonstrated modest antifungal effects (ElSohly et al., 1982). More recently, it proved to be an effective cytotoxic in high dosage on human epithelioid carcinoma (Baek et al., 1998), is the next most effective phytocannabinoid against breast cancer after CBD (Ligresti et al., 2006), is an antidepressant in the rodent tail suspension model (Musty and Deyo, 2006) and is a mildly anti-hypertensive agent (Maor et al., 2006). Additionally, CBG inhibits keratinocyte proliferation suggesting utility in psoriasis (Wilkinson and Williamson, 2007), it is a relatively potent TRPM8 antagonist for possible application in prostate cancer (De Petrocellis and Di Marzo, 2010) and detrusor over-activity and bladder pain (Mukerji et al., 2006). It is a strong AEA uptake inhibitor (De Petrocellis et al., 2011) and a powerful agent against MRSA (Appendino et al., 2008; vide infra). Finally, CBG behaves as a potent α-2 adrenoreceptor agonist, supporting analgesic effects previously noted (Formukong et al., 1988), and moderate 5-HT1A antagonist suggesting antidepressant properties (Cascio et al., 2010). Normally, CBG appears as a relatively low concentration intermediate in the plant, but recent breeding work has yielded cannabis chemotypes lacking in downstream enzymes that express 100% of their phytocannabinoid content as CBG (de Meijer and Hammond, 2005; de Meijer et al., 2009a).
THCV (Table 1) is a propyl analogue of THC, and can modulate intoxication of the latter, displaying 25% of its potency in early testing (Gill et al., 1970; Hollister, 1974). A recrudescence of interest accrues to this compound, which is a CB1 antagonist at lower doses (Thomas et al., 2005), but is a CB1 agonist at higher doses (Pertwee, 2008). THCV produces weight loss, decreased body fat and serum leptin concentrations with increased energy expenditure in obese mice (Cawthorne et al., 2007; Riedel et al., 2009). THCV also demonstrates prominent anticonvulsant properties in rodent cerebellum and pyriform cortex (Hill et al., 2010). THCV appears as a fractional component of many southern African cannabis chemotypes, although plants highly predominant in this agent have been produced (de Meijer, 2004). THCV recently demonstrated a CB2-based ability to suppress carageenan-induced hyperalgesia and inflammation, and both phases of formalin-induced pain behaviour via CB1 and CB2 in mice (Bolognini et al., 2010).
CBDV (Table 1), the propyl analogue of CBD, was first isolated in 1969 (Vollner et al., 1969), but formerly received little investigation. Pure CBDV inhibits diacylglycerol lipase [50% inhibitory concentration (IC50) 16.6 µM] and might decrease activity of its product, the endocannabinoid, 2-AG (De Petrocellis et al., 2011). It is also anticonvulsant in rodent hippocampal brain slices, comparable to phenobarbitone and felbamate (Jones et al., 2010).
Finally, CBN is a non-enzymatic oxidative by-product of THC, more prominent in aged cannabis samples (Merzouki and Mesa, 2002). It has a lower affinity for CB1 (Ki 211.2 nM) and CB2 (Ki 126.4 nM) (Rhee et al., 1997); and was judged inactive when tested alone in human volunteers, but produced greater sedation combined with THC (Musty et al., 1976). CBN demonstrated anticonvulsant (Turner et al., 1980), anti-inflammatory (Evans, 1991) and potent effects against MRSA (MIC 1 µg·mL−1). CBN is a TRPV2 (high-threshold thermosensor) agonist (EC 77.7 µM) of possible interest in treatment of burns (Qin et al., 2008). Like CBG, it inhibits keratinocyte proliferation (Wilkinson and Williamson, 2007), independently of cannabinoid receptor effects. CBN stimulates the recruitment of quiescent mesenchymal stem cells in marrow (10 µM), suggesting promotion of bone formation (Scutt and Williamson, 2007) and inhibits breast cancer resistance protein, albeit at a very high concentration (IC50 145 µM) (Holland et al., 2008).
Go to:
Cannabis terpenoids: neglected entourage compounds?
Terpenoids are EO components, previously conceived as the quintessential fifth element, ‘life force’ or spirit (Schmidt, 2010), and form the largest group of plant chemicals, with 15–20 000 fully characterized (Langenheim, 1994). Terpenoids, not cannabinoids, are responsible for the aroma of cannabis. Over 200 have been reported in the plant (Hendriks et al., 1975; 1977; Malingre et al., 1975; Davalos et al., 1977;Ross and ElSohly, 1996; Mediavilla and Steinemann, 1997; Rothschild et al., 2005; Brenneisen, 2007), but only a few studies have concentrated on their pharmacology (McPartland and Pruitt, 1999; McPartland and Mediavilla, 2001a; McPartland and Russo, 2001b). Their yield is less than 1% in most cannabis assays, but they may represent 10% of trichome content (Potter, 2009). Monoterpenes usually predominate (limonene, myrcene, pinene), but these headspace volatiles (Hood et al., 1973), while only lost at a rate of about 5% before processing (Gershenzon, 1994), do suffer diminished yields with drying and storage (Turner et al., 1980; Ross and ElSohly, 1996), resulting in a higher relative proportion of sesquiterpenoids (especially caryophyllene), as also often occurs in extracts. A ‘phytochemical polymorphism’ seems operative in the plant (Franz and Novak, 2010), as production favours agents such as limonene and pinene in flowers that are repellent to insects (Nerio et al., 2010), while lower fan leaves express higher concentrations of bitter sesquiterpenoids that act as anti-feedants for grazing animals (Potter, 2009). Evolutionarily, terpenoids seem to occur in complex and variable mixtures with marked structural diversity to serve various ecological roles. Terpenoid composition is under genetic control (Langenheim, 1994), and some enzymes produce multiple products, again supporting Mechoulam's ‘Law of Stinginess’. The particular mixture of mono- and sesquiterpenoids will determine viscosity, and in cannabis, this certainly is leveraged to practical advantage as the notable stickiness of cannabis exudations traps insects (McPartland et al., 2000), and thus, combined with the insecticidal phytocannabinoid acids (Sirikantaramas et al., 2005), provides a synergistic mechano-chemical defensive strategy versus predators.
As observed for cannabinoids, terpenoid production increases with light exposure, but decreases with soil fertility (Langenheim, 1994), and this is supported by the glasshouse experience that demonstrates higher yields if plants experience relative nitrogen lack just prior to harvest (Potter, 2004), favouring floral over foliar growth. EO composition is much more genetically than environmentally determined, however (Franz and Novak, 2010), and while cannabis is allogamous and normally requires repeat selective breeding for maintenance of quality, this problem may be practically circumvented by vegetative propagation of high-performance plants under controlled environmental conditions (light, heat and humidity) (Potter, 2009), and such techniques have proven to provide notable consistency to tight tolerances as Good Manufacturing Practice for any pharmaceutical would require (Fischedick et al., 2010).
The European Pharmacopoeia, Sixth Edition (2007), lists 28 EOs (Pauli and Schilcher, 2010). Terpenoids are pharmacologically versatile: they are lipophilic, interact with cell membranes, neuronal and muscle ion channels, neurotransmitter receptors, G-protein coupled (odorant) receptors, second messenger systems and enzymes (Bowles, 2003; Buchbauer, 2010). All the terpenoids discussed herein are Generally Recognized as Safe, as attested by the US Food and Drug Administration as food additives, or by the Food and Extract Manufacturers Association and other world regulatory bodies. Germane is the observation (Adams and Taylor, 2010) (p. 193), ‘With a high degree of confidence one may presume that EOs derived from food are likely to be safe’. Additionally, all the current entries are non-sensitizing to skin when fresh (Tisserand and Balacs, 1995; Adams and Taylor, 2010), but may cause allergic reactions at very low rates when oxidized (Matura et al., 2005). For additional pharmacological data on other common cannabis terpenoids not discussed herein (1,8-cineole, also known as eucalyptol, pulegone, α-terpineol, terpineol-4-ol, ρ-cymene, borneol and Δ-3-carene), please see McPartland and Russo (2001b).
Are cannabis terpenoids actually relevant to the effects of cannabis? Terpenoid components in concentrations above 0.05% are considered of pharmacological interest (Adams and Taylor, 2010). Animal studies are certainly supportive (Buchbauer et al., 1993). Mice exposed to terpenoid odours inhaled from ambient air for 1 h demonstrated profound effects on activity levels, suggesting a direct pharmacological effect on the brain, even at extremely low serum concentrations (examples: linalool with 73% reduction in motility at 4.22 ng·mL−1, pinene 13.77% increase at trace concentration, terpineol 45% reduction at 4.7 ng·mL−1). These levels are comparable to those of THC measured in humans receiving cannabis extracts yielding therapeutic effects in pain, or symptoms of multiple sclerosis in various randomized controlled trials (RCTs) (Russo, 2006; Huestis, 2007). Positive effects at undetectable serum concentrations with orange terpenes (primarily limonene, 35.25% increase in mouse activity), could be explainable on the basis of rapid redistribution and concentration in lipophilic cerebral structures. A similar rationale pertains to human studies (Komori et al., 1995), subsequently discussed. Limonene is highly bioavailable with 70% human pulmonary uptake (Falk-Filipsson et al., 1993), and a figure of 60% for pinene with rapid metabolism or redistribution (Falk et al., 1990). Ingestion and percutaneous absorption is also well documented in humans (Jäger et al., 1992): 1500 mg of lavender EO with 24.7% linalool (total 372 mg) was massaged into the skin of a 60 kg man for 10 min, resulting in a peak plasma concentration of 100 ng·mL−1 at 19 min, and a half-life of 13.76 min in serum (Jäger et al., 1992). EO mixtures (including limonene and pinene) also increase permeation of estradiol through mouse skin (Monti et al., 2002).
Government-approved cannabis supplied to patients in national programmes in the Netherlands and Canada is gamma-irradiated to sterilize coliform bacteria, but the safety of this technique for a smoked and inhaled product has never been specifically tested. Gamma-radiation significantly reduced linalool titres in fresh cilantro (Fan and Sokorai, 2002), and myrcene and linalool in orange juice (Fan and Gates, 2001).
d-limonene, common to the lemon and other citrus EOs (Table 2), is the second most widely distributed terpenoid in nature (Noma and Asakawa, 2010), and is the precursor to other monoterpenoids (Figure 2) through species-specific synthetic schemes. Unfortunately, these pathways have not yet been investigated in cannabis. The ubiquity of limonene serves, perhaps, as a demonstration of convergent evolution that supports an important ecological role for this monoterpene. Studies with varying methodology and dosing in citrus oils in mice suggest it to be a powerful anxiolytic agent (Carvalho-Freitas and Costa, 2002; Pultrini Ade et al., 2006), with one EO increasing serotonin in the prefrontal cortex, and dopamine (DA) in hippocampus mediated via 5-HT1A (Komiya et al., 2006). Compelling confirmatory evidence in humans was provided in a clinical study (Komori et al., 1995), in which hospitalized depressed patients were exposed to citrus fragrance in ambient air, with subsequent normalization of Hamilton Depression Scores, successful discontinuation of antidepressant medication in 9/12 patients and serum evidence of immune stimulation (CD4/8 ratio normalization). Limonene also produces apoptosis of breast cancer cells, and was employed at high doses in Phase II RCTs (Vigushin et al., 1998). Subsequent investigation in cancer treatment has centred on its immediate hepatic metabolite, perillic acid, which demonstrates anti-stress effects in rat brain (Fukumoto et al., 2008). A patent has been submitted, claiming that limonene effectively treats gastro-oesophageal reflux (Harris, 2010). Citrus EOs containing limonene proved effective against dermatophytes (Sanguinetti et al., 2007; Singh et al., 2010), and citrus EOs with terpenoid profiles resembling those in cannabis demonstrated strong radical scavenging properties (Choi et al., 2000). As noted above, limonene is highly bioavailable (Falk-Filipsson et al., 1993), and rapidly metabolized, but with indications of accumulation and retention in adipose tissues (e.g. brain). It is highly non-toxic (estimated human lethal dose 0.5–5 g·kg−1) and non-sensitizing (Von Burg, 1995)
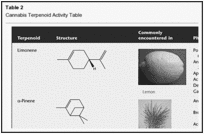
Table 2
Cannabis Terpenoid Activity Table
β-Myrcene is another common monoterpenoid in cannabis (Table 2) with myriad activities: diminishing inflammation via prostaglandin E-2 (PGE-2) (Lorenzetti et al., 1991), and blocking hepatic carcinogenesis by aflatoxin (De-Oliveira et al., 1997). Interestingly, myrcene is analgesic in mice, but this action can be blocked by naloxone, perhaps via the α-2 adrenoreceptor (Rao et al., 1990). It is non-mutagenic in the Ames test (Gomes-Carneiro et al., 2005). Myrcene is a recognized sedative as part of hops preparations (Humulus lupulus), employed to aid sleep in Germany (Bisset and Wichtl, 2004). Furthermore, myrcene acted as a muscle relaxant in mice, and potentiated barbiturate sleep time at high doses (do Vale et al., 2002). Together, these data would support the hypothesis that myrcene is a prominent sedative terpenoid in cannabis, and combined with THC, may produce the ‘couch-lock’ phenomenon of certain chemotypes that is alternatively decried or appreciated by recreational cannabis consumers.
α-Pinene is a bicyclic monoterpene (Table 2), and the most widely encountered terpenoid in nature (Noma and Asakawa, 2010). It appears in conifers and innumerable plant EOs, with an insect-repellent role. It is anti-inflammatory via PGE-1 (Gil et al., 1989), and is a bronchodilator in humans at low exposure levels (Falk et al., 1990). Pinene is a major component of Sideritis spp. (Kose et al., 2010) and Salvia spp. EOs (Ozek et al., 2010), both with prominent activity against MRSA (vide infra). Beyond this, it seems to be a broad-spectrum antibiotic (Nissen et al., 2010). α-Pinene forms the biosynthetic base for CB2 ligands, such as HU-308 (Hanus et al., 1999). Perhaps most compelling, however, is its activity as an acetylcholinesterase inhibitor aiding memory (Perry et al., 2000), with an observed IC50 of 0.44 mM (Miyazawa and Yamafuji, 2005). This feature could counteract short-term memory deficits induced by THC intoxication (vide infra).
D-Linalool is a monoterpenoid alcohol (Table 2), common to lavender (Lavandula angustifolia), whose psychotropic anxiolytic activity has been reviewed in detail (Russo, 2001). Interestingly, linalyl acetate, the other primary terpenoid in lavender, hydrolyses to linalool in gastric secretions (Bickers et al., 2003). Linalool proved sedating to mouse activity on inhalation (Buchbauer et al., 1991; Jirovetz et al., 1992). In traditional aromatherapy, linalool is the likely suspect in the remarkable therapeutic capabilities of lavender EO to alleviate skin burns without scarring (Gattefosse, 1993). Pertinent to this, the local anaesthetic effects of linalool (Re et al., 2000) are equal to those of procaine and menthol (Ghelardini et al., 1999). Another explanation would be its ability to produce hot-plate analgesia in mice (P < 0.001) that was reduced by administration of an adenosine A2A antagonist (Peana et al., 2006). It is also anti-nociceptive at high doses in mice via ionotropic glutamate receptors (Batista et al., 2008). Linalool demonstrated anticonvulsant and anti-glutamatergic activity (Elisabetsky et al., 1995), and reduced seizures as part of Ocimum basilicum EO after exposure to pentylenetetrazole, picrotoxin and strychnine (Ismail, 2006). Furthermore, linalool decreased K+-stimulated glutamate release and uptake in mouse synaptosomes (Silva Brum et al., 2001). These effects were summarized (Nunes et al., 2010, p. 303): ‘Overall, it seems reasonable to argue that the modulation of glutamate and GABA neurotransmitter systems are likely to be the critical mechanism responsible for the sedative, anxiolytic and anticonvulsant properties of linalool and EOs containing linalool in significant proportions’. Linalool also proved to be a powerful anti-leishmanial agent (do Socorro et al., 2003), and as a presumed lavender EO component, decreased morphine opioid usage after inhalation versus placebo (P = 0.04) in gastric banding in morbidly obese surgical patients (Kim et al., 2007).
β-Caryophyllene (Table 2) is generally the most common sesquiterpenoid encountered in cannabis (Mediavilla and Steinemann, 1997), wherein its evolutionary function may be due to its ability to attract insect predatory green lacewings, while simultaneously inhibiting insect herbivory (Langenheim, 1994). It is frequently the predominant terpenoid overall in cannabis extracts, particularly if they have been processed under heat for decarboxylation (Guy and Stott, 2005). Caryophyllene is common to black pepper (Piper nigrum) and Copaiba balsam (Copaifera officinalis) (Lawless, 1995). It is anti-inflammatory via PGE-1, comparable in potency to the toxic phenylbutazone (Basile et al., 1988), and an EO containing it was on par with etodolac and indomethacin (Ozturk and Ozbek, 2005). In contrast to the latter agents, however, caryophyllene was a gastric cytoprotective (Tambe et al., 1996), much as had been claimed in the past in treating duodenal ulcers in the UK with cannabis extract (Douthwaite, 1947). Caryophyllene may have contributed to antimalarial effects as an EO component (Campbell et al., 1997). Perhaps the greatest revelation regarding caryophyllene has been its demonstration as a selective full agonist at CB2 (100 nM), the first proven phytocannabinoid beyond the cannabis genus (Gertsch et al., 2008). Subsequent work has demonstrated that this dietary component produced anti-inflammatory analgesic activity at the lowest dose of 5 mg·kg−1 in wild-type, but not CB2 knockout mice (Gertsch, 2008). Given the lack of attributed psychoactivity of CB2 agonists, caryophyllene offers great promise as a therapeutic compound, whether systemically, or in dermatological applications such as contact dermatitis (Karsak et al., 2007). Sensitization reactions are quite rare, and probably due to oxidized product (Skold et al., 2006).
Nerolidol is a sesquiterpene alcohol with sedative properties (Binet et al., 1972), present as a low-level component in orange and other citrus peels (Table 2). It diminished experimentally induced formation of colon adenomas in rats (Wattenberg, 1991). It was an effective agent for enhancing skin penetration of 5-fluorouracil (Cornwell and Barry, 1994). This could be a helpful property in treating fungal growth, where it is also an inhibitor (Langenheim, 1994). It seems to have anti-protozoal parasite control benefits, as a potent antimalarial (Lopes et al., 1999; Rodrigues Goulart et al., 2004) and anti-leishmanial agent (Arruda et al., 2005). Nerolidol is non-toxic and non-sensitizing (Lapczynski et al., 2008).
Caryophyllene oxide (Table 2) is a sesquiterpenoid oxide common to lemon balm (Melissa officinalis), and to the eucalyptus, Melaleuca stypheloides, whose EO contains 43.8% (Farag et al., 2004). In the plant, it serves as an insecticidal/anti-feedant (Bettarini et al., 1993) and as broad-spectrum antifungal in plant defence (Langenheim, 1994). Analogously, the latter properties may prove therapeutic, as caryophyllene oxide demonstrated antifungal efficacy in a model of clinical onychomycosis comparable to ciclopiroxalamine and sulconazole, with an 8% concentration affecting eradication in 15 days (Yang et al., 1999). Caryophyllene oxide is non-toxic and non-sensitizing (Opdyke, 1983). This agent also demonstrates anti-platelet aggregation properties in vitro (Lin et al., 2003). Caryophyllene oxide has the distinction of being the component responsible for cannabis identification by drug-sniffing dogs (Stahl and Kunde, 1973).
Phytol (Table 2) is a diterpene (McGinty et al., 2010), present in cannabis extracts, as a breakdown product of chlorophyll and tocopherol. Phytol prevented vitamin A-induced teratogenesis by inhibiting conversion of retinol to a harmful metabolite, all-trans-retinoic acid (Arnhold et al., 2002). Phytol increased GABA expression via inhibition of succinic semialdehyde dehydrogenase, one of its degradative enzymes (Bang et al., 2002). Thus, the presence of phytol could account for the alleged relaxing effect of wild lettuce (Lactuca sativa), or green tea (Camellia sinensis), despite the latter's caffeine content.
Go to:
Selected possibilities for phytocannabinoid-terpenoid synergy
Cannabis and acne
AEA simulates lipid production in human sebocytes of sebaceous glands at low concentrations, but induces apoptosis at higher levels, suggesting that this system is under ECS control (Dobrosi et al., 2008). CBD 10–20 µM did not affect basal lipid synthesis in SZ95 sebocytes, but did block such stimulation by AEA and arachidonate (Biro et al., 2009). Higher doses of CBD (30–50 µM) induced sebocyte apoptosis, which was augmented in the presence of AEA. The effect of CBD to increase Ca++ was blocked by ruthenium red, a TRP-inhibitor. RNA-mediated silencing of TRPV1 and TRPV3 failed to attenuate CBD effects, but experiments did support the aetiological role of TRPV4, a putative regulator of systemic osmotic pressure (T. Bíró, 2010, pers. comm.). Given the observed ability of CBD to be absorbed transcutaneously, it offers great promise to attenuate the increased sebum production at the pathological root of acne.
Cannabis terpenoids could offer complementary activity. Two citrus EOs primarily composed of limonene inhibited Propionibacterium acnes, the key pathogen in acne (MIC 0.31 µL·mL−1), more potently than triclosan (Kim et al., 2008). Linalool alone demonstrated an MIC of 0.625 µL·mL−1. Both EOs inhibited P. acnes-induced TNF-α production, suggesting an adjunctive anti-inflammatory effect. In a similar manner, pinene was the most potent component of a tea-tree eucalyptus EO in suppression of P. acnes and Staph spp. in another report (Raman et al., 1995).
Considering the known minimal toxicities of CBD and these terpenoids and the above findings, new acne therapies utilizing whole CBD-predominant extracts, via multi-targeting (Wagner and Ulrich-Merzenich, 2009), may present a novel and promising therapeutic approach that poses minimal risks in comparison to isotretinoin.
Go to:
MRSA
MRSA accounted for 10% of cases of septicaemia and 18 650 deaths in the USA in 2005, a number greater than that attributable to human immunodeficiency virus/acquired immunodeficiency syndrome (Bancroft, 2007). Pure CBD and CBG powerfully inhibit MRSA (MIC 0.5–2 µg·mL−1) (Appendino et al., 2008).
Amongst terpenoids, pinene was a major component of Sideritis erythrantha EO that was as effective against MRSA and other antibiotic-resistant bacterial strains as vancomycin and other agents (Kose et al., 2010). ASalvia rosifolia EO with 34.8% pinene was also effective against MRSA (MIC 125 µg·mL−1). The ability of monoterpenoids to enhance skin permeability and entry of other drugs may further enhance antibiotic benefits (Wagner and Ulrich-Merzenich, 2009).
Given that CBG can be produced in selected cannabis chemotypes (de Meijer and Hammond, 2005; de Meijer et al., 2009a), with no residual THC as a possible drug abuse liability risk, a whole plant extract of a CBG-chemotype also expressing pinene would seem to offer an excellent, safe new antiseptic agent.
Go to:
Psychopharmacological applications: depression, anxiety, insomnia, dementia and addiction
Scientific investigation of the therapeutic application of terpenoids in psychiatry has been hampered by methodological concerns, subjective variability of results and a genuine dearth of appropriate randomized controlled studies of high quality (Russo, 2001; Bowles, 2003; Lis-Balchin, 2010). The same is true of phytocannabinoids (Fride and Russo, 2006). Abundant evidence supports the key role of the ECS in mediating depression (Hill and Gorzalka, 2005a,b;), as well as anxiety, whether induced by aversive stimuli, such as post-traumatic stress disorder (Marsicano et al., 2002) or pain (Hohmann et al., 2005), and psychosis (Giuffrida et al., 2004). With respect to the latter risk, the presence of CBD in smoked cannabis based on hair analysis seems to be a mitigating factor reducing its observed incidence (Morgan and Curran, 2008). A thorough review of cannabis and psychiatry is beyond the scope of this article, but several suggestions are offered with respect to possible therapeutic synergies operative with phytocannabinoids-terpenoid combinations. While the possible benefits of THC on depression remain controversial (Denson and Earleywine, 2006), much less worrisome would be CBD- or CBG-predominant preparations. Certainly the results obtained in human depression solely with a citrus scent (Komori et al., 1995), strongly suggest the possibility of synergistic benefit of a phytocannabinoid-terpenoid preparation. Enriched odour exposure in adult mice induced olfactory system neurogenesis (Rochefort et al., 2002), an intriguing result that could hypothetically support plasticity mechanisms in depression (Delgado and Moreno, 1999), and similar hypotheses with respect to the ECS in addiction treatment (Gerdeman and Lovinger, 2003). Phytocannabinoid-terpenoid synergy might theoretically apply.
The myriad effects of CBD on 5-HT1A activity provide a strong rationale for this and other phytocannabinoids as base compounds for treatment of anxiety. Newer findings, particularly imaging studies of CBD in normal individuals in anxiety models (Fusar-Poli et al., 2009; 2010; Crippa et al., 2010) support this hypothesis. Even more compelling is a recent randomized control trial of pure CBD in patients with social anxiety disorder with highly statistical improvements over placebo in anxiety and cognitive impairment (Crippa et al., 2011). Addition of anxiolytic limonene and linalool could contribute to the clinical efficacy of a CBD extract.
THC was demonstrated effective in a small crossover clinical trial versus placebo in 11 agitated dementia patients with Alzheimer's disease (Volicer et al., 1997). THC was also observed to be an acetylcholinesterase inhibitor in its own right, as well as preventing amyloid β-peptide aggregation in that disorder (Eubanks et al., 2006). Certainly, the anti-anxiety and anti-psychotic effects of CBD may be of additional benefit (Zuardiet al., 1991; 2006; Zuardi and Guimaraes, 1997). A recent study supports the concept that CBD, when present in significant proportion to THC, is capable of eliminating induced cognitive and memory deficits in normal subjects smoking cannabis (Morgan et al., 2010b). Furthermore, CBD may also have primary benefits on reduction of β-amyloid in Alzheimer's disease (Iuvone et al., 2004; Esposito et al., 2006a,b;). Psychopharmacological effects of limonene, pinene and linalool could putatively extend benefits in mood in such patients.
The effects of cannabis on sleep have been reviewed (Russo et al., 2007), and highlight the benefits that can accrue in this regard, particularly with respect to symptom reduction permitting better sleep, as opposed to a mere hypnotic effect. Certainly, terpenoids with pain-relieving, anti-anxiety or sedative effects may supplement such activity, notably, caryophyllene, linalool and myrcene.
The issue of cannabis addiction remains controversial. Some benefit of oral THC has been noted in cannabis withdrawal (Hart et al., 2002; Haney et al., 2004). More intriguing, perhaps, are claims of improvement on other substance dependencies, particularly cocaine (Labigalini et al., 1999; Dreher, 2002). The situation with CBD is yet more promising. CBD and THC at doses of 4 mg·kg−1 i.p. potentiated extinction of cocaine- and amphetamine-induced conditioned place preference in rats, and CBD produced no hedonic effects of its own (Parker et al., 2004). CBD 5 mg·kg−1·d−1 in rats attenuated heroin-seeking behaviour by conditioned stimuli, even after a lapse of 2 weeks (Ren et al., 2009). A suggested mechanism of CBD relates to its ability to reverse changes in α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate glutamate and CB1 receptor expression in the nucleus accumbens induced by heroin. The authors proposed CBD as a treatment for heroin craving and addiction relapse. A recent study demonstrated the fascinating result that patients with damage to the insula due to cerebrovascular accident were able to quit tobacco smoking without relapse or urges (Naqvi et al., 2007), highlighting this structure as a critical neural centre mediating addiction to nicotine. Further study has confirmed the role of the insula in cocaine, alcohol and heroin addiction (Naqvi and Bechara, 2009; Naqvi and Bechara, 2010). In a provocative parallel, CBD 600 mg p.o. was demonstrated to deactivate functional magnetic resonance imaging (fMRI) activity in human volunteers in the left insula versus placebo (P < 0.01) without accompanying sedation or psychoactive changes (Borgwardt et al., 2008), suggesting the possibility that CBD could act as a pharmaceutical surrogate for insular damage in exerting an anti-addiction therapeutic benefit. Human studies have recently demonstrated that human volunteers smoking cannabis with higher CBD content reduced their liking for drug-related stimuli, including food (Morgan et al., 2010a). The authors posited that CBD can modulate reinforcing properties of drugs of abuse, and help in training to reduce relapse to alcoholism. A single case report of a successful withdrawal from cannabis dependency utilizing pure CBD treatment was recently published (Crippa et al., 2010).
Perhaps terpenoids can provide adjunctive support. In a clinical trial, 48 cigarette smokers inhaling vapour from an EO of black pepper (Piper nigrum), a mint-menthol mixture or placebo (Rose and Behm, 1994). Black pepper EO reduced nicotine craving significantly (P < 0.01), an effect attributed to irritation of the bronchial tree, simulating the act of cigarette smoking, but without nicotine or actual burning of material. Rather, might not the effect have been pharmacological? The terpenoid profile of black pepper suggests possible candidates: myrcene via sedation, pinene via increased alertness, or especially caryophyllene via CB2 agonism and a newly discovered putative mechanism of action in addiction treatment.
CB2 is expressed in dopaminergic neurones in the ventral tegmental area and nucleus accumbens, areas mediating addictive phenomena (Xi et al., 2010). Activation of CB2 by the synthetic agonist JWH144 administered systemically, intranasally, or by microinjection into the nucleus accumbens in rats inhibited DA release and cocaine self-administration. Caryophyllene, as a high-potency selective CB2 agonist (Gertsch et al., 2008), would likely produce similar effects, and have the advantage of being a non-toxic dietary component. All factors considered, CBD, with caryophyllene, and possibly other adjunctive terpenoids in the extract, offers significant promise in future addiction treatment.
Go to:
Taming THC: cannabis entourage compounds as antidotes to intoxication
Various sources highlight the limited therapeutic index of pure THC, when given intravenously (D'Souza et al., 2004) or orally (Favrat et al., 2005), especially in people previously naïve to its effects. Acute overdose incidents involving THC or THC-predominant cannabis usually consist of self-limited panic reactions or toxic psychoses, for which no pharmacological intervention is generally necessary, and supportive counselling (reassurance or ‘talking down’) is sufficient to allow resolution without sequelae. CBD modulates the psychoactivity of THC and reduces its adverse event profile (Russo and Guy, 2006), highlighted by recent results above described. Could it be, however, that other cannabis components offer additional attenuation of the less undesirable effects of THC? History provides some clues.
In 10th century Persia, Al-Razi offered a prescription in his Manafi al-agdhiya wa-daf madarri-ha (p. 248), rendered (Lozano, 1993, p. 124; translation EBR) ‘– and to avoid these harms {from ingestion of cannabis seeds or hashish}, one should drink fresh water and ice or eat any acid fruits’. This concept was repeated in various forms by various authorities through the ages, including ibn Sina (ibn Sina (Avicenna), 1294), and Ibn al-Baytar (ibn al-Baytar, 1291), until O'Shaughnessy brought Indian hemp to Britain in 1843 (O'Shaughnessy, 1843). Robert Christison subsequently cited lemon (Figure 3A) as an antidote to acute intoxication in numerous cases (Christison, 1851) and this excerpt regarding morning-after residua (Christison, 1848) (p. 973):

Figure 3
Ancient cannabis antidotes. (A) Lemon (Citrus limon). (B) Calamus plant roots (Acorus calamus). (C) Pine nuts (Pinus spp.). (D) Black pepper (Piper nigrum).
Next morning there was an ordinary appetite, much torpidity, great defect and shortness of memory, extreme apparent protraction of time, but no peculiarity of articulation or other effect; and these symptoms lasted until 2 P.M., when they ceased entirely in a few minutes after taking lemonade.
Literary icons on both sides of the Atlantic espoused similar support for the citrus cure in the 19th century, notably Bayard Taylor after travels in Syria (Taylor, 1855), and Fitzhugh Ludlow after his voluntary experiments with ever higher cannabis extract doses in the USA (Ludlow, 1857). The sentiment was repeated by Calkins (1871), who noted the suggestion of a friend in Tunis that lemon retained the confidence of cure of overdoses by cannabis users in that region. This is supported by the observation that lemon juice, which normally contains small terpenoid titres, is traditionally enhanced in North Africa by the inclusion in drinks of the limonene-rich rind, as evidenced by the recipe for Agua Limón from modern Morocco (Morse and Mamane, 2001). In his comprehensive review of cannabis in the first half of the 20th century, Walton once more supported its prescription (Walton, 1938).
Another traditional antidote to cannabis employing Acorus calamus (Figure 3B) is evident from the Ayurvedic tradition of India (Lad, 1990, p. 131):
Calamus root is the best antidote for the ill effects of marijuana. . . . if one smokes a pinch of calamus root powder with the marijuana, this herb will completely neutralize the toxic side effects of the drug.
This claim has gained credence, not only through force of anecdotal accounts that abound on the Internet, but with formal scientific case reports and scientific analysis (McPartland et al., 2008) documenting clearer thinking and improved memory with the cannabis–calamus combination, and with provision of a scientific rationale: calamus contains beta-asarone, an acetylcholinesterase inhibitor with 10% of the potency of physotigmine (Mukherjee et al., 2007). Interestingly, the cannabis terpenoid, α-pinene, also has been characterized as a potent inhibitor of that enzyme (Miyazawa and Yamafuji, 2005), bolstering the hypothesis of a second antidote to THC contained in cannabis itself. Historical precedents also support pinene in this pharmacological role.
In the firstt century, Pliny wrote of cannabis in his Natural History, Book XXIV (Pliny, 1980, p. 164):
The gelotophyllis [‘leaves of laughter’ = cannabis] grows in Bactria and along the Borysthenes. If this be taken in myrrh and wine all kinds of phantoms beset the mind, causing laughter which persists until the kernels of pine-nuts are taken with pepper and honey in palm wine.
Of the components, palm wine is perhaps the most mysterious. Ethanol does not reduce cannabis intoxication (Mello and Mendelson, 1978). However, ancient wines were stored in clay pots or goatskins, and required preservation, usually with addition of pine tar or terebinth resin (from Pistacia spp.; McGovern et al., 2009). Pine tar is rich in pinene, as is terebinth resin (from Pistacia terebinthus; Tsokou et al., 2007), while the latter also contains limonene (Duru et al., 2003). Likewise, the pine nuts (Figure 3C) prescribed by Pliny the Elder harbour pinene, along with additional limonene (Salvadeo et al., 2007). Al-Ukbari also suggested pistachio nuts as a cannabis antidote in the 13th century (Lozano, 1993), and the ripe fruits of Pistacia terebinthussimilarly contain pinene (Couladis et al., 2003). The black pepper (Figure 3D), might offer the mental clarity afforded by pinene, sedation via myrcene and helpful contributions by β-caryophyllene. The historical suggestions for cannabis antidotes are thus supported by modern scientific rationales for the claims, and if proven experimentally would provide additional evidence of synergy (Berenbaum, 1989; Wagner and Ulrich-Merzenich, 2009).
Go to:
Conclusions and suggestions for future study
Considered ensemble, the preceding body of information supports the concept that selective breeding of cannabis chemotypes rich in ameliorative phytocannabinoid and terpenoid content offer complementary pharmacological activities that may strengthen and broaden clinical applications and improve the therapeutic index of cannabis extracts containing THC, or other base phytocannabinoids. Psychopharmacological and dermatological indications show the greatest promise.
One important remaining order of business is the elucidation of mono- and sesquiterpenoid biosynthetic pathways in cannabis, as has been achieved previously in other species of plants (Croteau, 1987; Gershenzon and Croteau, 1993; Bohlmann et al., 1998; Turner et al., 1999; Trapp and Croteau, 2001).
Various cannabis component combinations or cannabis extracts should be examined via high throughput pharmacological screening where not previously accomplished. Another goal is the investigation of the biochemical targets of the cannabis terpenoids, along with their mechanisms of action, particularly in the central nervous system. Possible techniques for such research include radio-labelling of select agents in animals with subsequent necropsy. On a molecular level, investigation of terpenoid changes to phytocannabinoid signal transduction and trafficking may prove illuminating. While it is known that terpenoids bind to odorant receptors in the nasal mucosa (Friedrich, 2004) and proximal olfactory structures (Barnea et al., 2004), it would be essential to ascertain if direct effects in limbic or other cerebral structures are operative. Given that farnesyl pyrophosphate is a sesquiterpenoid precursor and the most potent endogenous agonist yet discovered for GPR92 (McHugh et al., 2010), in silico studies attempting to match minor cannabinoids and terpenoids to orphan GPCRs may prove fruitful. Behavioural assays of agents in animal models may also provide clues. Simple combinations of phytocannabinoids and terpenoids may demonstrate synergy as antibiotics if MICs are appreciable lowered (Wagner and Ulrich-Merzenich, 2009). Ultimately, fMRI and single photon emission computed tomography studies in humans, with simultaneous drug reaction questionnaires and psychometric testing employing individual agents and phytocannabinoid-terpenoid pairings via vaporization or oromucosal application, would likely offer safe and effective methods to investigate possible interactions and synergy.
Should positive outcomes result from such studies, phytopharmaceutical development may follow. The development of zero-cannabinoid cannabis chemotypes (de Meijer et al., 2009b) has provided extracts that will facilitate discernment of the pharmacological effects and contributions of different fractions. Breeding work has already resulted in chemotypes that produce 97% of monoterpenoid content as myrcene, or 77% as limonene (E. de Meijer, pers. comm.). Selective cross-breeding of high-terpenoid- and high-phytocannabinoid-specific chemotypes has thus become a rational target that may lead to novel approaches to such disorders as treatment-resistant depression, anxiety, drug dependency, dementia and a panoply of dermatological disorders, as well as industrial applications as safer pesticides and antiseptics. A better future via cannabis phytochemistry may be an achievable goal through further research of the entourage effect in this versatile plant that may help it fulfil its promise as a pharmacological treasure trove.
Go to:
Acknowledgments
The author offers appreciation to the following individuals, who provided materials and/or consultation: David Potter, Etienne de Meijer, John McPartland, David Watson, Rob Clarke, Indalecio Lozano, Támas Bíró, José Crippa, Roger Pertwee, Colin Stott, Vincenzo Di Marzo, Luciano De Petrocellis, Patrick McGovern, John Riddle and Elisaldo Carlini. Most of all, I would like to thank Raphael Mechoulam for his example, guidance, friendship, a life of good works and for listening to many ‘crazy ideas’.
Go to:
Glossary
Abbreviations
2-AG 2-arachidonoylglycerol
5-HT 5-hydroxytryptamine (serotonin)
AD antidepressant
AEA arachidonoylethanolamide (anandamide)
AI anti-inflammatory
AMPA α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
Ca++ calcium ion
CB1/CB2 cannabinoid receptor 1 or 2
CBC cannabichromene
CBCA cannabichromenic acid
CBD cannabidiol
CBDA cannabidiolic acid
CBDV cannabidivarin
CBG cannabigerol
CBGA cannabigerolic acid
CBGV cannabigerivarin
CNS central nervous system
COX cyclo-oxygenase
DAGL diacylglycerol lipase
ECS endocannabinoid system
EO essential oil
FAAH fatty acid amidohydrolase
FDA US Food and Drug Administration
FEMA Food and Extract Manufacturers Association
fMRI functional magnetic resonance imaging
GABA gamma aminobutyric acid
GPCR G-protein coupled receptor
GPR G-protein coupled receptor
HEK human embryonic kidney
IC50 50% inhibitory concentration
i.p intraperitoneal
MAGL monoacylglycerol lipase
MIC minimum inhibitory concentration
MS multiple sclerosis
NGF nerve growth factor
NIDA US National Institute on Drug Abuse
PG prostaglandin
PTSD post-traumatic stress disorder
RCT randomized clinical trial
SPECT single photon emission computed tomography
SSADH succinic semialdehyde dehydrogenase
Sx symptoms
T1/2 half-life
TCA tricyclic antidepressant
THC tetrahydrocannabinol
THCA tetrahydrocannabinolic acid
THCV tetrahydrocannabivarin
TNF-α tumour necrosis factor-alpha, TRPV, transient receptor potential vanilloid receptor
Abstract
Tetrahydrocannabinol (THC) has been the primary focus of cannabis research since 1964, when Raphael Mechoulam isolated and synthesized it. More recently, the synergistic contributions of cannabidiol to cannabis pharmacology and analgesia have been scientifically demonstrated. Other phytocannabinoids, including tetrahydrocannabivarin, cannabigerol and cannabichromene, exert additional effects of therapeutic interest. Innovative conventional plant breeding has yielded cannabis chemotypes expressing high titres of each component for future study. This review will explore another echelon of phytotherapeutic agents, the cannabis terpenoids: limonene, myrcene, α-pinene, linalool, β-caryophyllene, caryophyllene oxide, nerolidol and phytol. Terpenoids share a precursor with phytocannabinoids, and are all flavour and fragrance components common to human diets that have been designated Generally Recognized as Safe by the US Food and Drug Administration and other regulatory agencies. Terpenoids are quite potent, and affect animal and even human behaviour when inhaled from ambient air at serum levels in the single digits ng·mL−1. They display unique therapeutic effects that may contribute meaningfully to the entourage effects of cannabis-based medicinal extracts. Particular focus will be placed on phytocannabinoid-terpenoid interactions that could produce synergy with respect to treatment of pain, inflammation, depression, anxiety, addiction, epilepsy, cancer, fungal and bacterial infections (including methicillin-resistant Staphylococcus aureus). Scientific evidence is presented for non-cannabinoid plant components as putative antidotes to intoxicating effects of THC that could increase its therapeutic index. Methods for investigating entourage effects in future experiments will be proposed. Phytocannabinoid-terpenoid synergy, if proven, increases the likelihood that an extensive pipeline of new therapeutic products is possible from this venerable plant.
LINKED ARTICLES
This article is part of a themed issue on Cannabinoids in Biology and Medicine. To view the other articles in this issue visit
Keywords: cannabinoids, terpenoids, essential oils, THC, CBD, limonene, pinene, linalool, caryophyllene, phytotherapy
Go to:
The roots of cannabis synergy
Cannabis has been a medicinal plant of unparalleled versatility for millennia (Mechoulam, 1986; Russo, 2007; 2008;), but whose mechanisms of action were an unsolved mystery until the discovery of tetrahydrocannabinol (THC) (Gaoni and Mechoulam, 1964a), the first cannabinoid receptor, CB1 (Devane et al., 1988), and the endocannabinoids, anandamide (arachidonoylethanolamide, AEA) (Devane et al., 1992) and 2-arachidonoylglycerol (2-AG) (Mechoulam et al., 1995; Sugiura et al., 1995). While a host of phytocannabinoids were discovered in the 1960s: cannabidiol (CBD) (Mechoulam and Shvo, 1963), cannabigerol (CBG) (Gaoni and Mechoulam, 1964b), cannabichromene (CBC) (Gaoni and Mechoulam, 1966), cannabidivarin (CBDV) (Vollner et al., 1969) and tetrahydrocannabivarin (THCV) (Gill et al., 1970), the overwhelming preponderance of research focused on psychoactive THC. Only recently has renewed interest been manifest in THC analogues, while other key components of the activity of cannabis and its extracts, the cannabis terpenoids, remain understudied (McPartland and Russo, 2001b; Russo and McPartland, 2003). The current review will reconsider essential oil (EO) agents, their peculiar pharmacology and possible therapeutic interactions with phytocannabinoids. Nomenclature follows conventions inAlexander et al. (2009).
Phytocannabinoids and terpenoids are synthesized in cannabis, in secretory cells inside glandular trichomes (Figure 1) that are most highly concentrated in unfertilized female flowers prior to senescence (Potter, 2004;Potter, 2009). Geranyl pyrophosphate is formed as a precursor via the deoxyxylulose pathway in cannabis (Fellermeier et al., 2001), and is a parent compound to both phytocannabinoids and terpenoids (Figure 2). After coupling with either olivetolic acid or divarinic acid, pentyl or propyl cannabinoid acids are produced, respectively, via enzymes that accept either substrate (de Meijer et al., 2003), a manifestation of Mechoulam's postulated ‘Nature's Law of Stinginess’. Although having important biochemical properties in their own right, acid forms of phytocannabinoids are most commonly decarboxylated via heat to produce the more familiar neutral phytocannabinoids (Table 1). Alternatively, geranyl pyrophosphate may form limonene and other monoterpenoids in secretory cell plastids, or couple with isopentenyl pyrophosphate in the cytoplasm to form farnesyl pyrophosphate, parent compound to the sesquiterpenoids, that co-localizes with transient receptor potential vanilloid receptor (TRPV) 1 in human dorsal root ganglion, suggesting a role in sensory processing of noxious stimuli (Bradshaw et al., 2009), and which is the most potent endogenous ligand to date on the G-protein coupled receptor (GPR) 92 (Oh et al., 2008).
Table 1
Phytocannabinoid activity table

Figure 1
Cannabis capitate glandular (EBR by permission of Bedrocan BV, Netherlands).

Figure 2
Phytocannabinoid and cannabis terpenoid biosynthesis.
An obvious question pertains to the chemical ecology of such syntheses that require obvious metabolic demands on the plant (Gershenzon, 1994), and these will be considered.
Is cannabis merely a crude vehicle for delivery of THC? Might it rather display herbal synergy (Williamson, 2001) encompassing potentiation of activity by active or inactive components, antagonism (evidenced by the ability of CBD to reduce side effects of THC; Russo and Guy, 2006), summation, pharmacokinetic and metabolic interactions? Recently, four basic mechanisms of synergy have been proposed (Wagner and Ulrich-Merzenich, 2009): (i) multi-target effects; (ii) pharmacokinetic effects such as improved solubility or bioavailability; (iii) agent interactions affecting bacterial resistance; and (iv) modulation of adverse events. Cannabis was cited as an illustration.
Could phytocannabinoids function analogously to the endocannabinoid system (ECS) with its combination of active and ‘inactive’ synergists, first described as an entourage (Ben-Shabat et al., 1998), with subsequent refinement (Mechoulam and Ben-Shabat, 1999) and qualification (p. 136): ‘This type of synergism may play a role in the widely held (but not experimentally based) view that in some cases plants are better drugs than the natural products isolated from them’. Support derives from studies in which cannabis extracts demonstrated effects two to four times greater than THC (Carlini et al., 1974); unidentified THC antagonists and synergists were claimed (Fairbairn and Pickens, 1981), anticonvulsant activity was observed beyond the cannabinoid fraction (Wilkinson et al., 2003), and extracts of THC and CBD modulated effects in hippocampal neurones distinctly from pure compounds (Ryan et al., 2006). Older literature also presented refutations: no observed differences were noted by humans ingesting or smoking pure THC versus herbal cannabis (Wachtel et al., 2002); pure THC seemed to account for all tetrad-type effects in mice (Varvel et al., 2005); and smoked cannabis with varying CBD or CBC content failed to yield subjective differences combined with THC (Ilan et al., 2005). Explanations include that the cannabis employed by Wachtel yielded 2.11% THC, but with only 0.3% cannabinol (CBN) and 0.05% CBD (Russo and McPartland, 2003), and Ilan's admission that CBN and CBD content might be too low to modulate THC. Another factor is apparent in that terpenoid yields from vaporization of street cannabis were 4.3–8.5 times of those from US National Institute on Drug Abuse cannabis (Bloor et al., 2008). It is undisputed that the black market cannabis in the UK (Potter et al., 2008), Continental Europe (King et al., 2005) and the USA (Mehmedic et al., 2010) has become almost exclusively a high-THC preparation to the almost total exclusion of other phytocannabinoids. If – as many consumers and experts maintain (Clarke, 2010) – there are biochemical, pharmacological and phenomenological distinctions between available cannabis ‘strains’, such phenomena are most likely related to relative terpenoid contents and ratios. This treatise will assess additional evidence for putative synergistic phytocannabinoid-terpenoid effects exclusive of THC, to ascertain whether this botanical may fulfil its promise as, ‘a neglected pharmacological treasure trove’ (Mechoulam, 2005).
Go to:
Phytocannabinoids, beyond THC: a brief survey
Phytocannabinoids are exclusively produced in cannabis (vide infra for exception), but their evolutionary and ecological raisons d'être were obscure until recently. THC production is maximized with increased light energy (Potter, 2009). It has been known for some time that CBG and CBC are mildly antifungal (ElSohly et al., 1982), as are THC and CBD against a cannabis pathogen (McPartland, 1984). More pertinent, however, is the mechanical stickiness of the trichomes, capable of trapping insects with all six legs (Potter, 2009). Tetrahydrocannabinolic acid (THCA) and cannabichromenic acid (Morimoto et al., 2007), as well as cannabidiolic acid and cannabigerolic acid (CBGA; Shoyama et al., 2008) produce necrosis in plant cells. Normally, the cannabinoid acids are sequestered in trichomes away from the flower tissues. Any trichome breakage at senescence may contribute to natural pruning of lower fan leaves that otherwise utilize energy that the plant preferentially diverts to the flower, in continued efforts to affect fertilization, generally in vain when subject to human horticulture for pharmaceutical production. THCA and CBGA have also proven to be insecticidal in their own right (Sirikantaramas et al., 2005).
Over 100 phytocannabinoids have been identified (Brenneisen, 2007; Mehmedic et al., 2010), but many are artefacts of analysis or are produced in trace quantities that have not permitted thorough investigation. The pharmacology of the more accessible phytocannabinoids has received excellent recent reviews (Pertwee et al., 2007; Izzo et al., 2009; De Petrocellis and Di Marzo, 2010; De Petrocellis et al., 2011), and will be summarized here, with emphasis on activities with particular synergistic potential.
THC (Table 1) is the most common phytocannabinoid in cannabis drug chemotypes, and is produced in the plant via an allele co-dominant with CBD (de Meijer et al., 2003). THC is a partial agonist at CB1 and cannabinoid receptor 2 (CB2) analogous to AEA, and underlying many of its activities as a psychoactive agent, analgesic, muscle relaxant and antispasmodic (Pacher et al., 2006). Additionally, it is a bronchodilator (Williams et al., 1976), neuroprotective antioxidant (Hampson et al., 1998), antipruritic agent in cholestatic jaundice (Neff et al., 2002) and has 20 times the anti-inflammatory power of aspirin and twice that of hydrocortisone (Evans, 1991). THC is likely to avoid potential pitfalls of either COX-1 or COX-2 inhibition, as such activity is only noted at concentrations far above those attained therapeutically (Stott et al., 2005).
CBD is the most common phytocannabinoid in fibre (hemp) plants, and second most prevalent in some drug chemotypes. It has proven extremely versatile pharmacologically (Table 1) (Pertwee, 2004; Mechoulam et al., 2007), displaying the unusual ability to antagonize CB1 at a low nM level in the presence of THC, despite having little binding affinity (Thomas et al., 2007), and supporting its modulatory effect on THC-associated adverse events such as anxiety, tachycardia, hunger and sedation in rats and humans (Nicholson et al., 2004; Murillo-Rodriguez et al., 2006; Russo and Guy, 2006). CBD is an analgesic (Costa et al., 2007), is a neuroprotective antioxidant more potent than ascorbate or tocopherol (Hampson et al., 1998), without COX inhibition (Stott et al., 2005), acts as a TRPV1 agonist analogous to capsaicin but without noxious effect (Bisogno et al., 2001), while also inhibiting uptake of AEA and weakly inhibiting its hydrolysis. CBD is an antagonist on GPR55, and also on GPR18, possibly supporting a therapeutic role in disorders of cell migration, notably endometriosis (McHugh et al., 2010). CBD is anticonvulsant (Carlini and Cunha, 1981;Jones et al., 2010), anti-nausea (Parker et al., 2002), cytotoxic in breast cancer (Ligresti et al., 2006) and many other cell lines while being cyto-preservative for normal cells (Parolaro and Massi, 2008), antagonizes tumour necrosis factor-alpha (TNF-α) in a rodent model of rheumatoid arthritis (Malfait et al., 2000), enhances adenosine receptor A2A signalling via inhibition of an adenosine transporter (Carrier et al., 2006), and prevents prion accumulation and neuronal toxicity (Dirikoc et al., 2007). A CBD extract showed greater anti-hyperalgesia over pure compound in a rat model with decreased allodynia, improved thermal perception and nerve growth factor levels and decreased oxidative damage (Comelli et al., 2009). CBD also displayed powerful activity against methicillin-resistant Staphylococcus aureus (MRSA), with a minimum inhibitory concentration (MIC) of 0.5–2 µg·mL−1 (Appendino et al., 2008). In 2005, it was demonstrated that CBD has agonistic activity at 5-hydroxytryptamine (5-HT)1A at 16 µM (Russo et al., 2005), and that despite the high concentration, may underlie its anti-anxiety activity (Resstel et al., 2009; Soares Vde et al., 2010), reduction of stroke risk (Mishima et al., 2005), anti-nausea effects (Rock et al., 2009) and ability to affect improvement in cognition in a mouse model of hepatic encephalopathy (Magen et al., 2009). A recent study has demonstrated that CBD 30 mg·kg−1 i.p. reduced immobility time in the forced swim test compared to imipramine (P < 0.01), an effect blocked by pre-treatment with the 5-HT1A antagonist WAY100635 (Zanelati et al., 2010), supporting a prospective role for CBD as an antidepressant. CBD also inhibits synthesis of lipids in sebocytes, and produces apoptosis at higher doses in a model of acne (vide infra). One example of CBD antagonism to THC would be the recent observation of lymphopenia in rats (CBD 5 mg·kg−1) mediated by possible CB2 inverse agonism (Ignatowska-Jankowska et al., 2009), an effect not reported in humans even at doses of pure CBD up to 800 mg (Crippa et al., 2010), possibly due to marked interspecies differences in CB2 sequences and signal transduction. CBD proved to be a critical factor in the ability of nabiximols oromucosal extract in successfully treating intractable cancer pain patients unresponsive to opioids (30% reduction in pain from baseline), as a high-THC extract devoid of CBD failed to distinguish from placebo (Johnson et al., 2010). This may represent true synergy if the THC–CBD combination were shown to provide a larger effect than a summation of those from the compounds separately (Berenbaum, 1989).
CBC (Table 1) was inactive on adenylate cyclase inhibition (Howlett, 1987), but showed activity in the mouse cannabinoid tetrad, but only at 100 mg·kg−1, and at a fraction of THC activity, via a non-CB1, non-CB2 mechanism (Delong et al., 2010). More pertinent are anti-inflammatory (Wirth et al., 1980) and analgesic activity (Davis and Hatoum, 1983), its ability to reduce THC intoxication in mice (Hatoum et al., 1981), antibiotic and antifungal effects (ElSohly et al., 1982), and observed cytotoxicity in cancer cell lines (Ligresti et al., 2006). A CBC-extract displayed pronounced antidepressant effect in rodent models (Deyo and Musty, 2003). Additionally, CBC was comparable to mustard oil in stimulating TRPA1-mediated Ca++in human embryonic kidney 293 cells (50–60 nM) (De Petrocellis et al., 2008). CBC recently proved to be a strong AEA uptake inhibitor (De Petrocellis et al., 2011). CBC production is normally maximal, earlier in the plant's life cycle (de Meijer et al., 2009a). An innovative technique employing cold water extraction of immature leaf matter from selectively bred cannabis chemotypes yields a high-CBC ‘enriched trichome preparation’ (Potter, 2009).
CBG (Table 1), the parent phytocannabinoid compound, has a relatively weak partial agonistic effect at CB1(Ki 440 nM) and CB2 (Ki 337 nM) (Gauson et al., 2007). Older work supports gamma aminobutyric acid (GABA) uptake inhibition greater than THC or CBD (Banerjee et al., 1975) that could suggest muscle relaxant properties. Analgesic and anti-erythemic effects and the ability to block lipooxygenase were said to surpass those of THC (Evans, 1991). CBG demonstrated modest antifungal effects (ElSohly et al., 1982). More recently, it proved to be an effective cytotoxic in high dosage on human epithelioid carcinoma (Baek et al., 1998), is the next most effective phytocannabinoid against breast cancer after CBD (Ligresti et al., 2006), is an antidepressant in the rodent tail suspension model (Musty and Deyo, 2006) and is a mildly anti-hypertensive agent (Maor et al., 2006). Additionally, CBG inhibits keratinocyte proliferation suggesting utility in psoriasis (Wilkinson and Williamson, 2007), it is a relatively potent TRPM8 antagonist for possible application in prostate cancer (De Petrocellis and Di Marzo, 2010) and detrusor over-activity and bladder pain (Mukerji et al., 2006). It is a strong AEA uptake inhibitor (De Petrocellis et al., 2011) and a powerful agent against MRSA (Appendino et al., 2008; vide infra). Finally, CBG behaves as a potent α-2 adrenoreceptor agonist, supporting analgesic effects previously noted (Formukong et al., 1988), and moderate 5-HT1A antagonist suggesting antidepressant properties (Cascio et al., 2010). Normally, CBG appears as a relatively low concentration intermediate in the plant, but recent breeding work has yielded cannabis chemotypes lacking in downstream enzymes that express 100% of their phytocannabinoid content as CBG (de Meijer and Hammond, 2005; de Meijer et al., 2009a).
THCV (Table 1) is a propyl analogue of THC, and can modulate intoxication of the latter, displaying 25% of its potency in early testing (Gill et al., 1970; Hollister, 1974). A recrudescence of interest accrues to this compound, which is a CB1 antagonist at lower doses (Thomas et al., 2005), but is a CB1 agonist at higher doses (Pertwee, 2008). THCV produces weight loss, decreased body fat and serum leptin concentrations with increased energy expenditure in obese mice (Cawthorne et al., 2007; Riedel et al., 2009). THCV also demonstrates prominent anticonvulsant properties in rodent cerebellum and pyriform cortex (Hill et al., 2010). THCV appears as a fractional component of many southern African cannabis chemotypes, although plants highly predominant in this agent have been produced (de Meijer, 2004). THCV recently demonstrated a CB2-based ability to suppress carageenan-induced hyperalgesia and inflammation, and both phases of formalin-induced pain behaviour via CB1 and CB2 in mice (Bolognini et al., 2010).
CBDV (Table 1), the propyl analogue of CBD, was first isolated in 1969 (Vollner et al., 1969), but formerly received little investigation. Pure CBDV inhibits diacylglycerol lipase [50% inhibitory concentration (IC50) 16.6 µM] and might decrease activity of its product, the endocannabinoid, 2-AG (De Petrocellis et al., 2011). It is also anticonvulsant in rodent hippocampal brain slices, comparable to phenobarbitone and felbamate (Jones et al., 2010).
Finally, CBN is a non-enzymatic oxidative by-product of THC, more prominent in aged cannabis samples (Merzouki and Mesa, 2002). It has a lower affinity for CB1 (Ki 211.2 nM) and CB2 (Ki 126.4 nM) (Rhee et al., 1997); and was judged inactive when tested alone in human volunteers, but produced greater sedation combined with THC (Musty et al., 1976). CBN demonstrated anticonvulsant (Turner et al., 1980), anti-inflammatory (Evans, 1991) and potent effects against MRSA (MIC 1 µg·mL−1). CBN is a TRPV2 (high-threshold thermosensor) agonist (EC 77.7 µM) of possible interest in treatment of burns (Qin et al., 2008). Like CBG, it inhibits keratinocyte proliferation (Wilkinson and Williamson, 2007), independently of cannabinoid receptor effects. CBN stimulates the recruitment of quiescent mesenchymal stem cells in marrow (10 µM), suggesting promotion of bone formation (Scutt and Williamson, 2007) and inhibits breast cancer resistance protein, albeit at a very high concentration (IC50 145 µM) (Holland et al., 2008).
Go to:
Cannabis terpenoids: neglected entourage compounds?
Terpenoids are EO components, previously conceived as the quintessential fifth element, ‘life force’ or spirit (Schmidt, 2010), and form the largest group of plant chemicals, with 15–20 000 fully characterized (Langenheim, 1994). Terpenoids, not cannabinoids, are responsible for the aroma of cannabis. Over 200 have been reported in the plant (Hendriks et al., 1975; 1977; Malingre et al., 1975; Davalos et al., 1977;Ross and ElSohly, 1996; Mediavilla and Steinemann, 1997; Rothschild et al., 2005; Brenneisen, 2007), but only a few studies have concentrated on their pharmacology (McPartland and Pruitt, 1999; McPartland and Mediavilla, 2001a; McPartland and Russo, 2001b). Their yield is less than 1% in most cannabis assays, but they may represent 10% of trichome content (Potter, 2009). Monoterpenes usually predominate (limonene, myrcene, pinene), but these headspace volatiles (Hood et al., 1973), while only lost at a rate of about 5% before processing (Gershenzon, 1994), do suffer diminished yields with drying and storage (Turner et al., 1980; Ross and ElSohly, 1996), resulting in a higher relative proportion of sesquiterpenoids (especially caryophyllene), as also often occurs in extracts. A ‘phytochemical polymorphism’ seems operative in the plant (Franz and Novak, 2010), as production favours agents such as limonene and pinene in flowers that are repellent to insects (Nerio et al., 2010), while lower fan leaves express higher concentrations of bitter sesquiterpenoids that act as anti-feedants for grazing animals (Potter, 2009). Evolutionarily, terpenoids seem to occur in complex and variable mixtures with marked structural diversity to serve various ecological roles. Terpenoid composition is under genetic control (Langenheim, 1994), and some enzymes produce multiple products, again supporting Mechoulam's ‘Law of Stinginess’. The particular mixture of mono- and sesquiterpenoids will determine viscosity, and in cannabis, this certainly is leveraged to practical advantage as the notable stickiness of cannabis exudations traps insects (McPartland et al., 2000), and thus, combined with the insecticidal phytocannabinoid acids (Sirikantaramas et al., 2005), provides a synergistic mechano-chemical defensive strategy versus predators.
As observed for cannabinoids, terpenoid production increases with light exposure, but decreases with soil fertility (Langenheim, 1994), and this is supported by the glasshouse experience that demonstrates higher yields if plants experience relative nitrogen lack just prior to harvest (Potter, 2004), favouring floral over foliar growth. EO composition is much more genetically than environmentally determined, however (Franz and Novak, 2010), and while cannabis is allogamous and normally requires repeat selective breeding for maintenance of quality, this problem may be practically circumvented by vegetative propagation of high-performance plants under controlled environmental conditions (light, heat and humidity) (Potter, 2009), and such techniques have proven to provide notable consistency to tight tolerances as Good Manufacturing Practice for any pharmaceutical would require (Fischedick et al., 2010).
The European Pharmacopoeia, Sixth Edition (2007), lists 28 EOs (Pauli and Schilcher, 2010). Terpenoids are pharmacologically versatile: they are lipophilic, interact with cell membranes, neuronal and muscle ion channels, neurotransmitter receptors, G-protein coupled (odorant) receptors, second messenger systems and enzymes (Bowles, 2003; Buchbauer, 2010). All the terpenoids discussed herein are Generally Recognized as Safe, as attested by the US Food and Drug Administration as food additives, or by the Food and Extract Manufacturers Association and other world regulatory bodies. Germane is the observation (Adams and Taylor, 2010) (p. 193), ‘With a high degree of confidence one may presume that EOs derived from food are likely to be safe’. Additionally, all the current entries are non-sensitizing to skin when fresh (Tisserand and Balacs, 1995; Adams and Taylor, 2010), but may cause allergic reactions at very low rates when oxidized (Matura et al., 2005). For additional pharmacological data on other common cannabis terpenoids not discussed herein (1,8-cineole, also known as eucalyptol, pulegone, α-terpineol, terpineol-4-ol, ρ-cymene, borneol and Δ-3-carene), please see McPartland and Russo (2001b).
Are cannabis terpenoids actually relevant to the effects of cannabis? Terpenoid components in concentrations above 0.05% are considered of pharmacological interest (Adams and Taylor, 2010). Animal studies are certainly supportive (Buchbauer et al., 1993). Mice exposed to terpenoid odours inhaled from ambient air for 1 h demonstrated profound effects on activity levels, suggesting a direct pharmacological effect on the brain, even at extremely low serum concentrations (examples: linalool with 73% reduction in motility at 4.22 ng·mL−1, pinene 13.77% increase at trace concentration, terpineol 45% reduction at 4.7 ng·mL−1). These levels are comparable to those of THC measured in humans receiving cannabis extracts yielding therapeutic effects in pain, or symptoms of multiple sclerosis in various randomized controlled trials (RCTs) (Russo, 2006; Huestis, 2007). Positive effects at undetectable serum concentrations with orange terpenes (primarily limonene, 35.25% increase in mouse activity), could be explainable on the basis of rapid redistribution and concentration in lipophilic cerebral structures. A similar rationale pertains to human studies (Komori et al., 1995), subsequently discussed. Limonene is highly bioavailable with 70% human pulmonary uptake (Falk-Filipsson et al., 1993), and a figure of 60% for pinene with rapid metabolism or redistribution (Falk et al., 1990). Ingestion and percutaneous absorption is also well documented in humans (Jäger et al., 1992): 1500 mg of lavender EO with 24.7% linalool (total 372 mg) was massaged into the skin of a 60 kg man for 10 min, resulting in a peak plasma concentration of 100 ng·mL−1 at 19 min, and a half-life of 13.76 min in serum (Jäger et al., 1992). EO mixtures (including limonene and pinene) also increase permeation of estradiol through mouse skin (Monti et al., 2002).
Government-approved cannabis supplied to patients in national programmes in the Netherlands and Canada is gamma-irradiated to sterilize coliform bacteria, but the safety of this technique for a smoked and inhaled product has never been specifically tested. Gamma-radiation significantly reduced linalool titres in fresh cilantro (Fan and Sokorai, 2002), and myrcene and linalool in orange juice (Fan and Gates, 2001).
d-limonene, common to the lemon and other citrus EOs (Table 2), is the second most widely distributed terpenoid in nature (Noma and Asakawa, 2010), and is the precursor to other monoterpenoids (Figure 2) through species-specific synthetic schemes. Unfortunately, these pathways have not yet been investigated in cannabis. The ubiquity of limonene serves, perhaps, as a demonstration of convergent evolution that supports an important ecological role for this monoterpene. Studies with varying methodology and dosing in citrus oils in mice suggest it to be a powerful anxiolytic agent (Carvalho-Freitas and Costa, 2002; Pultrini Ade et al., 2006), with one EO increasing serotonin in the prefrontal cortex, and dopamine (DA) in hippocampus mediated via 5-HT1A (Komiya et al., 2006). Compelling confirmatory evidence in humans was provided in a clinical study (Komori et al., 1995), in which hospitalized depressed patients were exposed to citrus fragrance in ambient air, with subsequent normalization of Hamilton Depression Scores, successful discontinuation of antidepressant medication in 9/12 patients and serum evidence of immune stimulation (CD4/8 ratio normalization). Limonene also produces apoptosis of breast cancer cells, and was employed at high doses in Phase II RCTs (Vigushin et al., 1998). Subsequent investigation in cancer treatment has centred on its immediate hepatic metabolite, perillic acid, which demonstrates anti-stress effects in rat brain (Fukumoto et al., 2008). A patent has been submitted, claiming that limonene effectively treats gastro-oesophageal reflux (Harris, 2010). Citrus EOs containing limonene proved effective against dermatophytes (Sanguinetti et al., 2007; Singh et al., 2010), and citrus EOs with terpenoid profiles resembling those in cannabis demonstrated strong radical scavenging properties (Choi et al., 2000). As noted above, limonene is highly bioavailable (Falk-Filipsson et al., 1993), and rapidly metabolized, but with indications of accumulation and retention in adipose tissues (e.g. brain). It is highly non-toxic (estimated human lethal dose 0.5–5 g·kg−1) and non-sensitizing (Von Burg, 1995)
Table 2
Cannabis Terpenoid Activity Table
β-Myrcene is another common monoterpenoid in cannabis (Table 2) with myriad activities: diminishing inflammation via prostaglandin E-2 (PGE-2) (Lorenzetti et al., 1991), and blocking hepatic carcinogenesis by aflatoxin (De-Oliveira et al., 1997). Interestingly, myrcene is analgesic in mice, but this action can be blocked by naloxone, perhaps via the α-2 adrenoreceptor (Rao et al., 1990). It is non-mutagenic in the Ames test (Gomes-Carneiro et al., 2005). Myrcene is a recognized sedative as part of hops preparations (Humulus lupulus), employed to aid sleep in Germany (Bisset and Wichtl, 2004). Furthermore, myrcene acted as a muscle relaxant in mice, and potentiated barbiturate sleep time at high doses (do Vale et al., 2002). Together, these data would support the hypothesis that myrcene is a prominent sedative terpenoid in cannabis, and combined with THC, may produce the ‘couch-lock’ phenomenon of certain chemotypes that is alternatively decried or appreciated by recreational cannabis consumers.
α-Pinene is a bicyclic monoterpene (Table 2), and the most widely encountered terpenoid in nature (Noma and Asakawa, 2010). It appears in conifers and innumerable plant EOs, with an insect-repellent role. It is anti-inflammatory via PGE-1 (Gil et al., 1989), and is a bronchodilator in humans at low exposure levels (Falk et al., 1990). Pinene is a major component of Sideritis spp. (Kose et al., 2010) and Salvia spp. EOs (Ozek et al., 2010), both with prominent activity against MRSA (vide infra). Beyond this, it seems to be a broad-spectrum antibiotic (Nissen et al., 2010). α-Pinene forms the biosynthetic base for CB2 ligands, such as HU-308 (Hanus et al., 1999). Perhaps most compelling, however, is its activity as an acetylcholinesterase inhibitor aiding memory (Perry et al., 2000), with an observed IC50 of 0.44 mM (Miyazawa and Yamafuji, 2005). This feature could counteract short-term memory deficits induced by THC intoxication (vide infra).
D-Linalool is a monoterpenoid alcohol (Table 2), common to lavender (Lavandula angustifolia), whose psychotropic anxiolytic activity has been reviewed in detail (Russo, 2001). Interestingly, linalyl acetate, the other primary terpenoid in lavender, hydrolyses to linalool in gastric secretions (Bickers et al., 2003). Linalool proved sedating to mouse activity on inhalation (Buchbauer et al., 1991; Jirovetz et al., 1992). In traditional aromatherapy, linalool is the likely suspect in the remarkable therapeutic capabilities of lavender EO to alleviate skin burns without scarring (Gattefosse, 1993). Pertinent to this, the local anaesthetic effects of linalool (Re et al., 2000) are equal to those of procaine and menthol (Ghelardini et al., 1999). Another explanation would be its ability to produce hot-plate analgesia in mice (P < 0.001) that was reduced by administration of an adenosine A2A antagonist (Peana et al., 2006). It is also anti-nociceptive at high doses in mice via ionotropic glutamate receptors (Batista et al., 2008). Linalool demonstrated anticonvulsant and anti-glutamatergic activity (Elisabetsky et al., 1995), and reduced seizures as part of Ocimum basilicum EO after exposure to pentylenetetrazole, picrotoxin and strychnine (Ismail, 2006). Furthermore, linalool decreased K+-stimulated glutamate release and uptake in mouse synaptosomes (Silva Brum et al., 2001). These effects were summarized (Nunes et al., 2010, p. 303): ‘Overall, it seems reasonable to argue that the modulation of glutamate and GABA neurotransmitter systems are likely to be the critical mechanism responsible for the sedative, anxiolytic and anticonvulsant properties of linalool and EOs containing linalool in significant proportions’. Linalool also proved to be a powerful anti-leishmanial agent (do Socorro et al., 2003), and as a presumed lavender EO component, decreased morphine opioid usage after inhalation versus placebo (P = 0.04) in gastric banding in morbidly obese surgical patients (Kim et al., 2007).
β-Caryophyllene (Table 2) is generally the most common sesquiterpenoid encountered in cannabis (Mediavilla and Steinemann, 1997), wherein its evolutionary function may be due to its ability to attract insect predatory green lacewings, while simultaneously inhibiting insect herbivory (Langenheim, 1994). It is frequently the predominant terpenoid overall in cannabis extracts, particularly if they have been processed under heat for decarboxylation (Guy and Stott, 2005). Caryophyllene is common to black pepper (Piper nigrum) and Copaiba balsam (Copaifera officinalis) (Lawless, 1995). It is anti-inflammatory via PGE-1, comparable in potency to the toxic phenylbutazone (Basile et al., 1988), and an EO containing it was on par with etodolac and indomethacin (Ozturk and Ozbek, 2005). In contrast to the latter agents, however, caryophyllene was a gastric cytoprotective (Tambe et al., 1996), much as had been claimed in the past in treating duodenal ulcers in the UK with cannabis extract (Douthwaite, 1947). Caryophyllene may have contributed to antimalarial effects as an EO component (Campbell et al., 1997). Perhaps the greatest revelation regarding caryophyllene has been its demonstration as a selective full agonist at CB2 (100 nM), the first proven phytocannabinoid beyond the cannabis genus (Gertsch et al., 2008). Subsequent work has demonstrated that this dietary component produced anti-inflammatory analgesic activity at the lowest dose of 5 mg·kg−1 in wild-type, but not CB2 knockout mice (Gertsch, 2008). Given the lack of attributed psychoactivity of CB2 agonists, caryophyllene offers great promise as a therapeutic compound, whether systemically, or in dermatological applications such as contact dermatitis (Karsak et al., 2007). Sensitization reactions are quite rare, and probably due to oxidized product (Skold et al., 2006).
Nerolidol is a sesquiterpene alcohol with sedative properties (Binet et al., 1972), present as a low-level component in orange and other citrus peels (Table 2). It diminished experimentally induced formation of colon adenomas in rats (Wattenberg, 1991). It was an effective agent for enhancing skin penetration of 5-fluorouracil (Cornwell and Barry, 1994). This could be a helpful property in treating fungal growth, where it is also an inhibitor (Langenheim, 1994). It seems to have anti-protozoal parasite control benefits, as a potent antimalarial (Lopes et al., 1999; Rodrigues Goulart et al., 2004) and anti-leishmanial agent (Arruda et al., 2005). Nerolidol is non-toxic and non-sensitizing (Lapczynski et al., 2008).
Caryophyllene oxide (Table 2) is a sesquiterpenoid oxide common to lemon balm (Melissa officinalis), and to the eucalyptus, Melaleuca stypheloides, whose EO contains 43.8% (Farag et al., 2004). In the plant, it serves as an insecticidal/anti-feedant (Bettarini et al., 1993) and as broad-spectrum antifungal in plant defence (Langenheim, 1994). Analogously, the latter properties may prove therapeutic, as caryophyllene oxide demonstrated antifungal efficacy in a model of clinical onychomycosis comparable to ciclopiroxalamine and sulconazole, with an 8% concentration affecting eradication in 15 days (Yang et al., 1999). Caryophyllene oxide is non-toxic and non-sensitizing (Opdyke, 1983). This agent also demonstrates anti-platelet aggregation properties in vitro (Lin et al., 2003). Caryophyllene oxide has the distinction of being the component responsible for cannabis identification by drug-sniffing dogs (Stahl and Kunde, 1973).
Phytol (Table 2) is a diterpene (McGinty et al., 2010), present in cannabis extracts, as a breakdown product of chlorophyll and tocopherol. Phytol prevented vitamin A-induced teratogenesis by inhibiting conversion of retinol to a harmful metabolite, all-trans-retinoic acid (Arnhold et al., 2002). Phytol increased GABA expression via inhibition of succinic semialdehyde dehydrogenase, one of its degradative enzymes (Bang et al., 2002). Thus, the presence of phytol could account for the alleged relaxing effect of wild lettuce (Lactuca sativa), or green tea (Camellia sinensis), despite the latter's caffeine content.
Go to:
Selected possibilities for phytocannabinoid-terpenoid synergy
Cannabis and acne
AEA simulates lipid production in human sebocytes of sebaceous glands at low concentrations, but induces apoptosis at higher levels, suggesting that this system is under ECS control (Dobrosi et al., 2008). CBD 10–20 µM did not affect basal lipid synthesis in SZ95 sebocytes, but did block such stimulation by AEA and arachidonate (Biro et al., 2009). Higher doses of CBD (30–50 µM) induced sebocyte apoptosis, which was augmented in the presence of AEA. The effect of CBD to increase Ca++ was blocked by ruthenium red, a TRP-inhibitor. RNA-mediated silencing of TRPV1 and TRPV3 failed to attenuate CBD effects, but experiments did support the aetiological role of TRPV4, a putative regulator of systemic osmotic pressure (T. Bíró, 2010, pers. comm.). Given the observed ability of CBD to be absorbed transcutaneously, it offers great promise to attenuate the increased sebum production at the pathological root of acne.
Cannabis terpenoids could offer complementary activity. Two citrus EOs primarily composed of limonene inhibited Propionibacterium acnes, the key pathogen in acne (MIC 0.31 µL·mL−1), more potently than triclosan (Kim et al., 2008). Linalool alone demonstrated an MIC of 0.625 µL·mL−1. Both EOs inhibited P. acnes-induced TNF-α production, suggesting an adjunctive anti-inflammatory effect. In a similar manner, pinene was the most potent component of a tea-tree eucalyptus EO in suppression of P. acnes and Staph spp. in another report (Raman et al., 1995).
Considering the known minimal toxicities of CBD and these terpenoids and the above findings, new acne therapies utilizing whole CBD-predominant extracts, via multi-targeting (Wagner and Ulrich-Merzenich, 2009), may present a novel and promising therapeutic approach that poses minimal risks in comparison to isotretinoin.
Go to:
MRSA
MRSA accounted for 10% of cases of septicaemia and 18 650 deaths in the USA in 2005, a number greater than that attributable to human immunodeficiency virus/acquired immunodeficiency syndrome (Bancroft, 2007). Pure CBD and CBG powerfully inhibit MRSA (MIC 0.5–2 µg·mL−1) (Appendino et al., 2008).
Amongst terpenoids, pinene was a major component of Sideritis erythrantha EO that was as effective against MRSA and other antibiotic-resistant bacterial strains as vancomycin and other agents (Kose et al., 2010). ASalvia rosifolia EO with 34.8% pinene was also effective against MRSA (MIC 125 µg·mL−1). The ability of monoterpenoids to enhance skin permeability and entry of other drugs may further enhance antibiotic benefits (Wagner and Ulrich-Merzenich, 2009).
Given that CBG can be produced in selected cannabis chemotypes (de Meijer and Hammond, 2005; de Meijer et al., 2009a), with no residual THC as a possible drug abuse liability risk, a whole plant extract of a CBG-chemotype also expressing pinene would seem to offer an excellent, safe new antiseptic agent.
Go to:
Psychopharmacological applications: depression, anxiety, insomnia, dementia and addiction
Scientific investigation of the therapeutic application of terpenoids in psychiatry has been hampered by methodological concerns, subjective variability of results and a genuine dearth of appropriate randomized controlled studies of high quality (Russo, 2001; Bowles, 2003; Lis-Balchin, 2010). The same is true of phytocannabinoids (Fride and Russo, 2006). Abundant evidence supports the key role of the ECS in mediating depression (Hill and Gorzalka, 2005a,b;), as well as anxiety, whether induced by aversive stimuli, such as post-traumatic stress disorder (Marsicano et al., 2002) or pain (Hohmann et al., 2005), and psychosis (Giuffrida et al., 2004). With respect to the latter risk, the presence of CBD in smoked cannabis based on hair analysis seems to be a mitigating factor reducing its observed incidence (Morgan and Curran, 2008). A thorough review of cannabis and psychiatry is beyond the scope of this article, but several suggestions are offered with respect to possible therapeutic synergies operative with phytocannabinoids-terpenoid combinations. While the possible benefits of THC on depression remain controversial (Denson and Earleywine, 2006), much less worrisome would be CBD- or CBG-predominant preparations. Certainly the results obtained in human depression solely with a citrus scent (Komori et al., 1995), strongly suggest the possibility of synergistic benefit of a phytocannabinoid-terpenoid preparation. Enriched odour exposure in adult mice induced olfactory system neurogenesis (Rochefort et al., 2002), an intriguing result that could hypothetically support plasticity mechanisms in depression (Delgado and Moreno, 1999), and similar hypotheses with respect to the ECS in addiction treatment (Gerdeman and Lovinger, 2003). Phytocannabinoid-terpenoid synergy might theoretically apply.
The myriad effects of CBD on 5-HT1A activity provide a strong rationale for this and other phytocannabinoids as base compounds for treatment of anxiety. Newer findings, particularly imaging studies of CBD in normal individuals in anxiety models (Fusar-Poli et al., 2009; 2010; Crippa et al., 2010) support this hypothesis. Even more compelling is a recent randomized control trial of pure CBD in patients with social anxiety disorder with highly statistical improvements over placebo in anxiety and cognitive impairment (Crippa et al., 2011). Addition of anxiolytic limonene and linalool could contribute to the clinical efficacy of a CBD extract.
THC was demonstrated effective in a small crossover clinical trial versus placebo in 11 agitated dementia patients with Alzheimer's disease (Volicer et al., 1997). THC was also observed to be an acetylcholinesterase inhibitor in its own right, as well as preventing amyloid β-peptide aggregation in that disorder (Eubanks et al., 2006). Certainly, the anti-anxiety and anti-psychotic effects of CBD may be of additional benefit (Zuardiet al., 1991; 2006; Zuardi and Guimaraes, 1997). A recent study supports the concept that CBD, when present in significant proportion to THC, is capable of eliminating induced cognitive and memory deficits in normal subjects smoking cannabis (Morgan et al., 2010b). Furthermore, CBD may also have primary benefits on reduction of β-amyloid in Alzheimer's disease (Iuvone et al., 2004; Esposito et al., 2006a,b;). Psychopharmacological effects of limonene, pinene and linalool could putatively extend benefits in mood in such patients.
The effects of cannabis on sleep have been reviewed (Russo et al., 2007), and highlight the benefits that can accrue in this regard, particularly with respect to symptom reduction permitting better sleep, as opposed to a mere hypnotic effect. Certainly, terpenoids with pain-relieving, anti-anxiety or sedative effects may supplement such activity, notably, caryophyllene, linalool and myrcene.
The issue of cannabis addiction remains controversial. Some benefit of oral THC has been noted in cannabis withdrawal (Hart et al., 2002; Haney et al., 2004). More intriguing, perhaps, are claims of improvement on other substance dependencies, particularly cocaine (Labigalini et al., 1999; Dreher, 2002). The situation with CBD is yet more promising. CBD and THC at doses of 4 mg·kg−1 i.p. potentiated extinction of cocaine- and amphetamine-induced conditioned place preference in rats, and CBD produced no hedonic effects of its own (Parker et al., 2004). CBD 5 mg·kg−1·d−1 in rats attenuated heroin-seeking behaviour by conditioned stimuli, even after a lapse of 2 weeks (Ren et al., 2009). A suggested mechanism of CBD relates to its ability to reverse changes in α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate glutamate and CB1 receptor expression in the nucleus accumbens induced by heroin. The authors proposed CBD as a treatment for heroin craving and addiction relapse. A recent study demonstrated the fascinating result that patients with damage to the insula due to cerebrovascular accident were able to quit tobacco smoking without relapse or urges (Naqvi et al., 2007), highlighting this structure as a critical neural centre mediating addiction to nicotine. Further study has confirmed the role of the insula in cocaine, alcohol and heroin addiction (Naqvi and Bechara, 2009; Naqvi and Bechara, 2010). In a provocative parallel, CBD 600 mg p.o. was demonstrated to deactivate functional magnetic resonance imaging (fMRI) activity in human volunteers in the left insula versus placebo (P < 0.01) without accompanying sedation or psychoactive changes (Borgwardt et al., 2008), suggesting the possibility that CBD could act as a pharmaceutical surrogate for insular damage in exerting an anti-addiction therapeutic benefit. Human studies have recently demonstrated that human volunteers smoking cannabis with higher CBD content reduced their liking for drug-related stimuli, including food (Morgan et al., 2010a). The authors posited that CBD can modulate reinforcing properties of drugs of abuse, and help in training to reduce relapse to alcoholism. A single case report of a successful withdrawal from cannabis dependency utilizing pure CBD treatment was recently published (Crippa et al., 2010).
Perhaps terpenoids can provide adjunctive support. In a clinical trial, 48 cigarette smokers inhaling vapour from an EO of black pepper (Piper nigrum), a mint-menthol mixture or placebo (Rose and Behm, 1994). Black pepper EO reduced nicotine craving significantly (P < 0.01), an effect attributed to irritation of the bronchial tree, simulating the act of cigarette smoking, but without nicotine or actual burning of material. Rather, might not the effect have been pharmacological? The terpenoid profile of black pepper suggests possible candidates: myrcene via sedation, pinene via increased alertness, or especially caryophyllene via CB2 agonism and a newly discovered putative mechanism of action in addiction treatment.
CB2 is expressed in dopaminergic neurones in the ventral tegmental area and nucleus accumbens, areas mediating addictive phenomena (Xi et al., 2010). Activation of CB2 by the synthetic agonist JWH144 administered systemically, intranasally, or by microinjection into the nucleus accumbens in rats inhibited DA release and cocaine self-administration. Caryophyllene, as a high-potency selective CB2 agonist (Gertsch et al., 2008), would likely produce similar effects, and have the advantage of being a non-toxic dietary component. All factors considered, CBD, with caryophyllene, and possibly other adjunctive terpenoids in the extract, offers significant promise in future addiction treatment.
Go to:
Taming THC: cannabis entourage compounds as antidotes to intoxication
Various sources highlight the limited therapeutic index of pure THC, when given intravenously (D'Souza et al., 2004) or orally (Favrat et al., 2005), especially in people previously naïve to its effects. Acute overdose incidents involving THC or THC-predominant cannabis usually consist of self-limited panic reactions or toxic psychoses, for which no pharmacological intervention is generally necessary, and supportive counselling (reassurance or ‘talking down’) is sufficient to allow resolution without sequelae. CBD modulates the psychoactivity of THC and reduces its adverse event profile (Russo and Guy, 2006), highlighted by recent results above described. Could it be, however, that other cannabis components offer additional attenuation of the less undesirable effects of THC? History provides some clues.
In 10th century Persia, Al-Razi offered a prescription in his Manafi al-agdhiya wa-daf madarri-ha (p. 248), rendered (Lozano, 1993, p. 124; translation EBR) ‘– and to avoid these harms {from ingestion of cannabis seeds or hashish}, one should drink fresh water and ice or eat any acid fruits’. This concept was repeated in various forms by various authorities through the ages, including ibn Sina (ibn Sina (Avicenna), 1294), and Ibn al-Baytar (ibn al-Baytar, 1291), until O'Shaughnessy brought Indian hemp to Britain in 1843 (O'Shaughnessy, 1843). Robert Christison subsequently cited lemon (Figure 3A) as an antidote to acute intoxication in numerous cases (Christison, 1851) and this excerpt regarding morning-after residua (Christison, 1848) (p. 973):

Figure 3
Ancient cannabis antidotes. (A) Lemon (Citrus limon). (B) Calamus plant roots (Acorus calamus). (C) Pine nuts (Pinus spp.). (D) Black pepper (Piper nigrum).
Next morning there was an ordinary appetite, much torpidity, great defect and shortness of memory, extreme apparent protraction of time, but no peculiarity of articulation or other effect; and these symptoms lasted until 2 P.M., when they ceased entirely in a few minutes after taking lemonade.
Literary icons on both sides of the Atlantic espoused similar support for the citrus cure in the 19th century, notably Bayard Taylor after travels in Syria (Taylor, 1855), and Fitzhugh Ludlow after his voluntary experiments with ever higher cannabis extract doses in the USA (Ludlow, 1857). The sentiment was repeated by Calkins (1871), who noted the suggestion of a friend in Tunis that lemon retained the confidence of cure of overdoses by cannabis users in that region. This is supported by the observation that lemon juice, which normally contains small terpenoid titres, is traditionally enhanced in North Africa by the inclusion in drinks of the limonene-rich rind, as evidenced by the recipe for Agua Limón from modern Morocco (Morse and Mamane, 2001). In his comprehensive review of cannabis in the first half of the 20th century, Walton once more supported its prescription (Walton, 1938).
Another traditional antidote to cannabis employing Acorus calamus (Figure 3B) is evident from the Ayurvedic tradition of India (Lad, 1990, p. 131):
Calamus root is the best antidote for the ill effects of marijuana. . . . if one smokes a pinch of calamus root powder with the marijuana, this herb will completely neutralize the toxic side effects of the drug.
This claim has gained credence, not only through force of anecdotal accounts that abound on the Internet, but with formal scientific case reports and scientific analysis (McPartland et al., 2008) documenting clearer thinking and improved memory with the cannabis–calamus combination, and with provision of a scientific rationale: calamus contains beta-asarone, an acetylcholinesterase inhibitor with 10% of the potency of physotigmine (Mukherjee et al., 2007). Interestingly, the cannabis terpenoid, α-pinene, also has been characterized as a potent inhibitor of that enzyme (Miyazawa and Yamafuji, 2005), bolstering the hypothesis of a second antidote to THC contained in cannabis itself. Historical precedents also support pinene in this pharmacological role.
In the firstt century, Pliny wrote of cannabis in his Natural History, Book XXIV (Pliny, 1980, p. 164):
The gelotophyllis [‘leaves of laughter’ = cannabis] grows in Bactria and along the Borysthenes. If this be taken in myrrh and wine all kinds of phantoms beset the mind, causing laughter which persists until the kernels of pine-nuts are taken with pepper and honey in palm wine.
Of the components, palm wine is perhaps the most mysterious. Ethanol does not reduce cannabis intoxication (Mello and Mendelson, 1978). However, ancient wines were stored in clay pots or goatskins, and required preservation, usually with addition of pine tar or terebinth resin (from Pistacia spp.; McGovern et al., 2009). Pine tar is rich in pinene, as is terebinth resin (from Pistacia terebinthus; Tsokou et al., 2007), while the latter also contains limonene (Duru et al., 2003). Likewise, the pine nuts (Figure 3C) prescribed by Pliny the Elder harbour pinene, along with additional limonene (Salvadeo et al., 2007). Al-Ukbari also suggested pistachio nuts as a cannabis antidote in the 13th century (Lozano, 1993), and the ripe fruits of Pistacia terebinthussimilarly contain pinene (Couladis et al., 2003). The black pepper (Figure 3D), might offer the mental clarity afforded by pinene, sedation via myrcene and helpful contributions by β-caryophyllene. The historical suggestions for cannabis antidotes are thus supported by modern scientific rationales for the claims, and if proven experimentally would provide additional evidence of synergy (Berenbaum, 1989; Wagner and Ulrich-Merzenich, 2009).
Go to:
Conclusions and suggestions for future study
Considered ensemble, the preceding body of information supports the concept that selective breeding of cannabis chemotypes rich in ameliorative phytocannabinoid and terpenoid content offer complementary pharmacological activities that may strengthen and broaden clinical applications and improve the therapeutic index of cannabis extracts containing THC, or other base phytocannabinoids. Psychopharmacological and dermatological indications show the greatest promise.
One important remaining order of business is the elucidation of mono- and sesquiterpenoid biosynthetic pathways in cannabis, as has been achieved previously in other species of plants (Croteau, 1987; Gershenzon and Croteau, 1993; Bohlmann et al., 1998; Turner et al., 1999; Trapp and Croteau, 2001).
Various cannabis component combinations or cannabis extracts should be examined via high throughput pharmacological screening where not previously accomplished. Another goal is the investigation of the biochemical targets of the cannabis terpenoids, along with their mechanisms of action, particularly in the central nervous system. Possible techniques for such research include radio-labelling of select agents in animals with subsequent necropsy. On a molecular level, investigation of terpenoid changes to phytocannabinoid signal transduction and trafficking may prove illuminating. While it is known that terpenoids bind to odorant receptors in the nasal mucosa (Friedrich, 2004) and proximal olfactory structures (Barnea et al., 2004), it would be essential to ascertain if direct effects in limbic or other cerebral structures are operative. Given that farnesyl pyrophosphate is a sesquiterpenoid precursor and the most potent endogenous agonist yet discovered for GPR92 (McHugh et al., 2010), in silico studies attempting to match minor cannabinoids and terpenoids to orphan GPCRs may prove fruitful. Behavioural assays of agents in animal models may also provide clues. Simple combinations of phytocannabinoids and terpenoids may demonstrate synergy as antibiotics if MICs are appreciable lowered (Wagner and Ulrich-Merzenich, 2009). Ultimately, fMRI and single photon emission computed tomography studies in humans, with simultaneous drug reaction questionnaires and psychometric testing employing individual agents and phytocannabinoid-terpenoid pairings via vaporization or oromucosal application, would likely offer safe and effective methods to investigate possible interactions and synergy.
Should positive outcomes result from such studies, phytopharmaceutical development may follow. The development of zero-cannabinoid cannabis chemotypes (de Meijer et al., 2009b) has provided extracts that will facilitate discernment of the pharmacological effects and contributions of different fractions. Breeding work has already resulted in chemotypes that produce 97% of monoterpenoid content as myrcene, or 77% as limonene (E. de Meijer, pers. comm.). Selective cross-breeding of high-terpenoid- and high-phytocannabinoid-specific chemotypes has thus become a rational target that may lead to novel approaches to such disorders as treatment-resistant depression, anxiety, drug dependency, dementia and a panoply of dermatological disorders, as well as industrial applications as safer pesticides and antiseptics. A better future via cannabis phytochemistry may be an achievable goal through further research of the entourage effect in this versatile plant that may help it fulfil its promise as a pharmacological treasure trove.
Go to:
Acknowledgments
The author offers appreciation to the following individuals, who provided materials and/or consultation: David Potter, Etienne de Meijer, John McPartland, David Watson, Rob Clarke, Indalecio Lozano, Támas Bíró, José Crippa, Roger Pertwee, Colin Stott, Vincenzo Di Marzo, Luciano De Petrocellis, Patrick McGovern, John Riddle and Elisaldo Carlini. Most of all, I would like to thank Raphael Mechoulam for his example, guidance, friendship, a life of good works and for listening to many ‘crazy ideas’.
Go to:
Glossary
Abbreviations
2-AG 2-arachidonoylglycerol
5-HT 5-hydroxytryptamine (serotonin)
AD antidepressant
AEA arachidonoylethanolamide (anandamide)
AI anti-inflammatory
AMPA α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
Ca++ calcium ion
CB1/CB2 cannabinoid receptor 1 or 2
CBC cannabichromene
CBCA cannabichromenic acid
CBD cannabidiol
CBDA cannabidiolic acid
CBDV cannabidivarin
CBG cannabigerol
CBGA cannabigerolic acid
CBGV cannabigerivarin
CNS central nervous system
COX cyclo-oxygenase
DAGL diacylglycerol lipase
ECS endocannabinoid system
EO essential oil
FAAH fatty acid amidohydrolase
FDA US Food and Drug Administration
FEMA Food and Extract Manufacturers Association
fMRI functional magnetic resonance imaging
GABA gamma aminobutyric acid
GPCR G-protein coupled receptor
GPR G-protein coupled receptor
HEK human embryonic kidney
IC50 50% inhibitory concentration
i.p intraperitoneal
MAGL monoacylglycerol lipase
MIC minimum inhibitory concentration
MS multiple sclerosis
NGF nerve growth factor
NIDA US National Institute on Drug Abuse
PG prostaglandin
PTSD post-traumatic stress disorder
RCT randomized clinical trial
SPECT single photon emission computed tomography
SSADH succinic semialdehyde dehydrogenase
Sx symptoms
T1/2 half-life
TCA tricyclic antidepressant
THC tetrahydrocannabinol
THCA tetrahydrocannabinolic acid
THCV tetrahydrocannabivarin
TNF-α tumour necrosis factor-alpha, TRPV, transient receptor potential vanilloid receptor





