Frankster
Never trust a doctor who's plants have died.
Supporter
- 5,188
- 313
Sucrose, fructan, and starch metabolic pathways engage with central metabolic pools in a two-way process. Synthesis of these end-products draws from UDP glucose (UDPG) and/or hexose phosphate substrate; whilst their breakdown resupplies this pool. Trehalose 6-phosphate (T6P) is also made from this pool as a signal of carbon availability. T6P inhibits the feast–famine protein kinase, SnRK1. Inhibition of SnRK1 by T6P is associated with down-regulation of photosynthesis and reduced levels of T6P with up-regulation of photosynthesis. Synthesis and storage of a new end-product in the form of oil (triacylglycerol) by DGAT/cys-OLE expression draws carbon out of the UDPG/hexose phosphate pool in a one-way process. This reduces metabolically available carbon and the amount of T6P, which enables the activation of photosynthesis for longer.
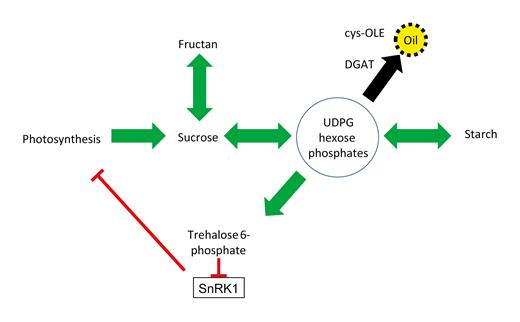
The findings suggest that engineering carbon sequestration in sink tissues in the form of triacylglycerol presents an interesting new tool to address this question as well as providing a useful technology for enhancing both biomass and energy densification of crops.
Photorespiration in plants Photorespiration is a multi-organellar process in photosynthetic cells involving the chloroplast (green), peroxisome (blue), mitochondria (yellow), and cytosol (white). Known transporters between organelles are depicted. For every two oxygenation reactions catalyzed by Rubisco in the chloroplast, one molecule of glycerate is generated in the peroxisome and transported to the chloroplast for reintroduction into the C3 cycle and one carbon is released as CO2 in the mitochondria. Number of carbons per molecule are indicated. Energy demand of photorespiration depicted in reducing equivalents (NAD(P)H) and ATP.
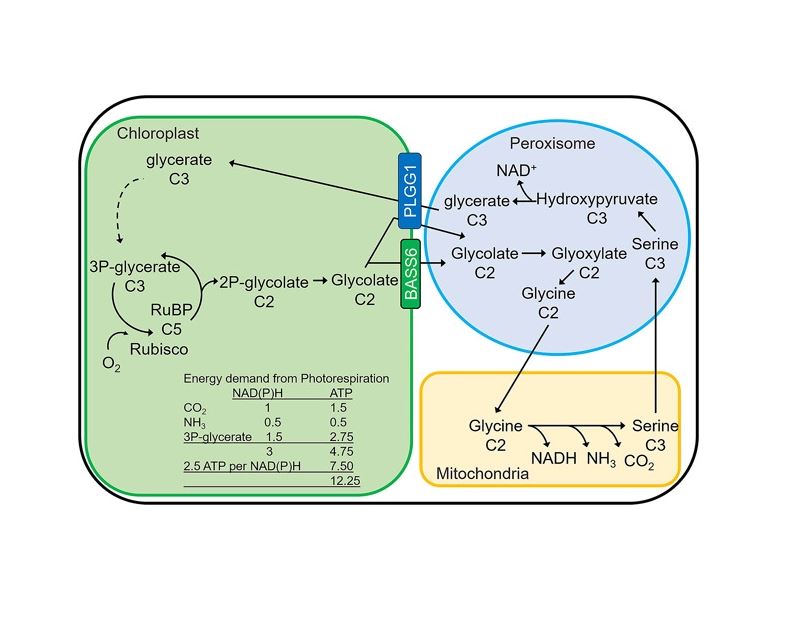
The primary carboxylase of the C3 photosynthetic cycle is ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco), which generates two molecules of 3-phosphoglycerate (3-PGA) by catalyzing the addition of CO2 to the five-carbon acceptor, ribulose-1,5-bisphosphate (RuBP). A major inefficiency of the C3 cycle occurs when Rubisco catalyzes oxygenation of RuBP, which results in the generation of one molecule of 3-PGA and one molecule of 2-phosphoglycolate
Three main approaches have been taken to lower the cost of photorespiration with the goal of increasing plant productivity. The first is to reduce oxygenation of RuBP by increasing the efficiency of Rubisco through either genetic manipulation of the enzyme, or by concentrating CO2 around Rubisco. The second is to manipulate the native photorespiratory pathway through gene mutation or overexpression to increase the rate of toxic byproduct recycling and carbon recovery . Lastly, the third approach is to install non-native alternative metabolic pathways to reduce the energetic cost of photorespiration (Figure 2)
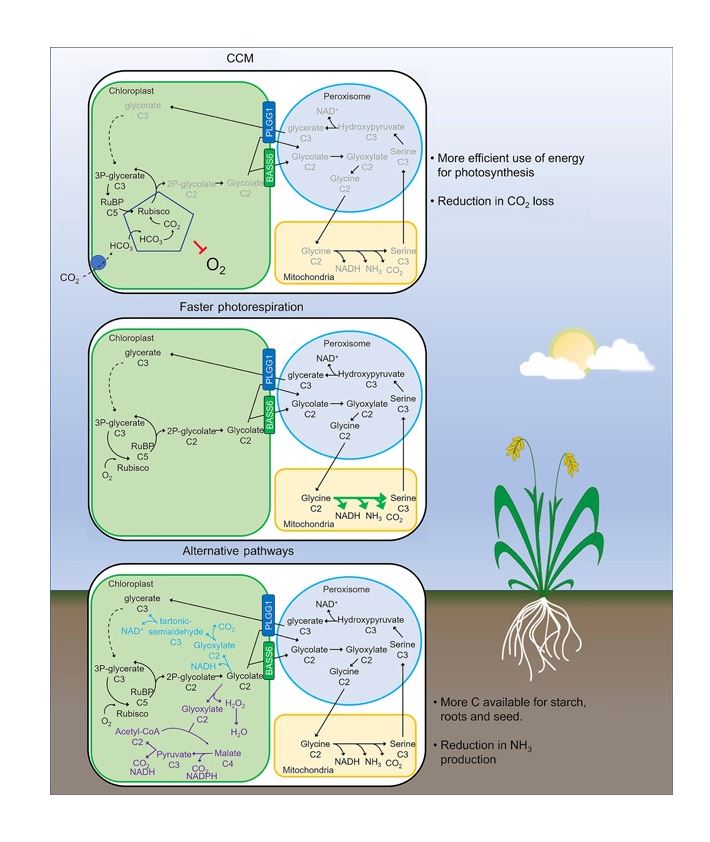
An alternative method to increase CO2 concentration at Rubisco is to introduce C4 photosynthesis into C3 crops. C4 photosynthesis has independently evolved from C3 photosynthesis over 60 times and is thought to be an adaption to higher photorespiratory pressures. Most C4 plant species are located in the grasslands of tropical and subtropical regions around the world.
The CO2 concentration near Rubisco occurs in C4 plants by dividing photosynthesis activities between the mesophyll and bundle sheath cells. In mesophyll cells, CO2 is first converted into four-carbon malate by the non-oxygen sensitive PEP carboxylase. This four-carbon dicarboxylic acid is then actively transported into the bundle sheath where it is decarboxylated, increasing the CO2 concentration near Rubisco. This C4 “CO2 pump” requires two additional ATPs for every mole of CO2 fixed. In addition, introduction of C4 photosynthesis into C3 plants requires directed changes in both the biochemistry of photosynthesis and leaf structure with increased photosynthetically active bundle sheath cells, although single cell C4 photosynthesis and C3-C4 intermediates could also be sources of engineering strategies (Matsuoka et al. 2001; Schuler et al. 2016). Currently, there has been some success in engineering C4 photosynthesis into rice through the C4Rice project, but further investigation into how C4 photosynthesis evolves and the regulatory elements needed to significantly convert C3 photosynthesis to C4 is needed to fully realize the benefits in crops
The findings suggest that engineering carbon sequestration in sink tissues in the form of triacylglycerol presents an interesting new tool to address this question as well as providing a useful technology for enhancing both biomass and energy densification of crops.
Photorespiration in plants Photorespiration is a multi-organellar process in photosynthetic cells involving the chloroplast (green), peroxisome (blue), mitochondria (yellow), and cytosol (white). Known transporters between organelles are depicted. For every two oxygenation reactions catalyzed by Rubisco in the chloroplast, one molecule of glycerate is generated in the peroxisome and transported to the chloroplast for reintroduction into the C3 cycle and one carbon is released as CO2 in the mitochondria. Number of carbons per molecule are indicated. Energy demand of photorespiration depicted in reducing equivalents (NAD(P)H) and ATP.
The primary carboxylase of the C3 photosynthetic cycle is ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco), which generates two molecules of 3-phosphoglycerate (3-PGA) by catalyzing the addition of CO2 to the five-carbon acceptor, ribulose-1,5-bisphosphate (RuBP). A major inefficiency of the C3 cycle occurs when Rubisco catalyzes oxygenation of RuBP, which results in the generation of one molecule of 3-PGA and one molecule of 2-phosphoglycolate
Three main approaches have been taken to lower the cost of photorespiration with the goal of increasing plant productivity. The first is to reduce oxygenation of RuBP by increasing the efficiency of Rubisco through either genetic manipulation of the enzyme, or by concentrating CO2 around Rubisco. The second is to manipulate the native photorespiratory pathway through gene mutation or overexpression to increase the rate of toxic byproduct recycling and carbon recovery . Lastly, the third approach is to install non-native alternative metabolic pathways to reduce the energetic cost of photorespiration (Figure 2)
Alternate Rubiscos
Rubisco has long been a target of genetic manipulation, with the goal of improving its selectivity and kinetic performanceConcentrating carbon near Rubisco
In addition to modifying Rubisco directly, other approaches aim to decrease oxygenation reactions by concentrating CO2 within the chloroplast. One strategy to increase the concentration of CO2 around Rubisco uses non-plant carbon concentrating mechanisms (CCMs) (Figure 2).An alternative method to increase CO2 concentration at Rubisco is to introduce C4 photosynthesis into C3 crops. C4 photosynthesis has independently evolved from C3 photosynthesis over 60 times and is thought to be an adaption to higher photorespiratory pressures. Most C4 plant species are located in the grasslands of tropical and subtropical regions around the world.
The CO2 concentration near Rubisco occurs in C4 plants by dividing photosynthesis activities between the mesophyll and bundle sheath cells. In mesophyll cells, CO2 is first converted into four-carbon malate by the non-oxygen sensitive PEP carboxylase. This four-carbon dicarboxylic acid is then actively transported into the bundle sheath where it is decarboxylated, increasing the CO2 concentration near Rubisco. This C4 “CO2 pump” requires two additional ATPs for every mole of CO2 fixed. In addition, introduction of C4 photosynthesis into C3 plants requires directed changes in both the biochemistry of photosynthesis and leaf structure with increased photosynthetically active bundle sheath cells, although single cell C4 photosynthesis and C3-C4 intermediates could also be sources of engineering strategies (Matsuoka et al. 2001; Schuler et al. 2016). Currently, there has been some success in engineering C4 photosynthesis into rice through the C4Rice project, but further investigation into how C4 photosynthesis evolves and the regulatory elements needed to significantly convert C3 photosynthesis to C4 is needed to fully realize the benefits in crops




