Desertboy
- 1,416
- 263
I quoted this before but I believe it to be very important!
Photoperiodism in a Short-Day Plant
 Experiments with the cocklebur have shown that the term short-day is something of a misnomer; what the cocklebur needs is a sufficiently long night.
Experiments with the cocklebur have shown that the term short-day is something of a misnomer; what the cocklebur needs is a sufficiently long night.
Phytochrome
http://users.rcn.com/jkimball.me a.ultranet/BiologyPages/P/Photoperiodism.html
This contradicts the 660nm not causing Pr-Prf they effectively kept a plant in veg using a pulsing light mid point through of 660nm.
They also forced flower plants under less dark hours using pulsing FR and pretty much proved for me at least that pulsing FR during the dark hours will eliminate hermies and mean you can visit during lights off with any nm light without interrupting the flower cycle as long as the pulses are close enough together (Every 15mins for 1-5 seconds sounds good to me).
This study to me is the validation of the theory and proof that it does work even if the plant in question wasn't cannabis.
Photoperiodism in a Short-Day Plant

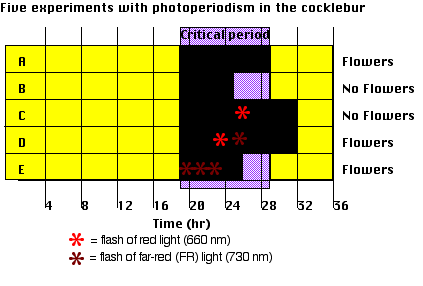
- Cockleburs (adapted to the latitude of Michigan) will flower only if they have been kept in the dark for at least 8.5 hours — the critical period. (A and B).
- Interruption of an otherwise long night by light — red (660 nm) rays are particularly effective — prevents flowering. (C) unless
- it is followed by irradiation with far red (730 nm) light (D). See spectrum of electromagnetic radiation.
- An intense exposure to far red light at the start of the night reduces the dark requirement by 2 hours (E).
Phytochrome
- Phytochrome is a homodimer: two identical protein molecules each conjugated to a light-absorbing molecule (compare rhodopsin).
- Plants make 5 phytochromes: PhyA, PhyB, as well as C, D, and E.
- There is some redundancy in function of the different phytochromes, but there also seem to be functions that are unique to one or another. The phytochromes also differ in their absorption spectrum; that is, which wavelengths (e.g., red vs. far-red) they absorb best.
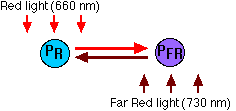
- Phytochromes exist in two interconvertible forms
- PR because it absorbs red (R; 660 nm) light;
- PFR because it absorbs far red (FR; 730 nm) light.
- These are the relationships:
- Absorption of red light by PR converts it into PFR.
- Absorption of far red light by PFR converts it into PR.
- In the dark, PFR spontaneously converts back to PR.
http://users.rcn.com/jkimball.me a.ultranet/BiologyPages/P/Photoperiodism.html
This contradicts the 660nm not causing Pr-Prf they effectively kept a plant in veg using a pulsing light mid point through of 660nm.
They also forced flower plants under less dark hours using pulsing FR and pretty much proved for me at least that pulsing FR during the dark hours will eliminate hermies and mean you can visit during lights off with any nm light without interrupting the flower cycle as long as the pulses are close enough together (Every 15mins for 1-5 seconds sounds good to me).
This study to me is the validation of the theory and proof that it does work even if the plant in question wasn't cannabis.





