jumpincactus
Premium Member
Supporter
- 11,609
- 438
@philbuYes, always use good, cross-referenced, ph pen, also periodically use the soil testing kit, the mix's pH is 7, and to refresh my memory with my excel sheet, the nuted water has been 6.3. According to the dutchmaster site you kindly offered, correct range is 5.9 to 6.5. Again, seems I'm in range and I would def consider raising pH above what's recommended if I had a clearer understanding as to why.
Thanks!
Over the years I have started using the practice of allowing a wide swing in my ph ranges. Timing will vary due to crop cycle, mediums used etc. I honestly believe that those growers that set to one specific ph range and never allow it to swing from low to high seem to experience more ph related nute deficiencies. For example, if I am running hydro ebb n flo the recommended range of my selected nute line is 5.2 - 6.0. So when mixing up a rez I will start at 5.2 to 5.3 and allow it to swing on up up thru 6.0. In my mind this allows for a more complete uptake of all the essential minerals and elements.
When I was always running a solid 5.8 and never let it drift was when I had the most problems. Thats just my 2 c and the method I use.
You might also want to check on your water sources alkalinity as well, because the alkalinity of your water will also factor in on your ph levels. Alkalinity doesn't seem to be discussed much and it is certainly a player in the whole water chemistry thing. Your Kh levels have a direct correlation to the buffering capacity of your water/ medium.
Heres a more detailed explanation...... The ‘K’ in KH comes from the German word 'karbonate'. KH is a measure of bicarbonate (HCO3-) and carbonate (CO32-) ions that act as buffers in the water to prevent the pH dropping or changing sharply (especially at night if you have plants in the aquarium). One degree KH is equal to 17.9 mg/I (ppm) CaCO3. It's also measured in degrees. The degree symbol may be replaced with a d (ie. 2 dKH)
you can do your own Kh test using a Kh test kit from any decent aquarium supply store. No need to send water out to a lab.
Here is a link that will explain alkalinity and ph better than I can. Take what you need and leave the rest. Peace
Had to remove the pdf file it was linking to my docs on my puter. not sure how that works. Heres a paste of it lets see if that works.
WATER ALKALINITY vs. pH – WHAT’S THE DIFFERENCE
Water alkalinity and pH are not the same. Water pH measures the amount of hydrogen (acid ions) in the
water, whereas water alkalinity is a measure of the carbonate and bicarbonate levels in water. Think of
carbonates and bicarbonates as dissolved limestone. The higher the alkalinity of the water, the more
lime it contains and therefore, the more rapidly the water can cause the growing medium pH to rise. On
the other hand, the pH of the water does not have any influence on the pH of the growing medium.
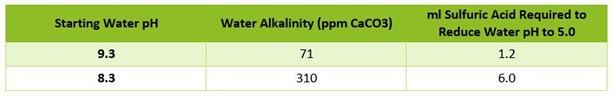
For example, the chart above shows the starting pH of two water sources and the amount of acid
required for each to reach a pH of 5.0. The water with the higher pH did not require as much as acid as
the one with the lower starting pH. At first glance, this may not make sense. However, note the
alkalinity in the center column. Regardless of the starting pH, the higher the alkalinity of the water
source, the more acid is required to reduce pH to 5.0. For all water sources, it is the alkalinity that
actually determines how much acid to use, not the pH.
How does water alkalinity influence the pH of the growing medium?
In the graph below, vinca plugs were grown for 49 days and constant fed with the same fertilizer at 125
ppm. The plugs were irrigated with three different water sources with varying alkalinities. The pH rose
in all three media samples partially due to the fertilizer used and the limestone in the growing medium.
However, the higher the alkalinity of the water source, the higher the pH of the growing medium.
This graph shows influence of the water
alkalinity on the pH of vinca plugs after 49
days. Notice the higher the water alkalinity,
the higher the pH of the growing medium rose
after 49 days.
Data taken from: D. Bailey & P.
V. Nelson, Substrate pH and Water Quality.
1996 Ohio Short Course
It is clear that the pH of the water and the alkalinity are not the same. In fact, the pH of the water does
not dictate the pH of the growing medium, but in fact it is the alkalinity of the water source that
influences the pH of the growing medium. This is important for growers to know since alkalinity has
significant impact on growing medium pH, choosing the correct fertilizer(s) and injecting acid, when
applicable.
Last edited: