RFT
- 119
- 43
I believe nitrogen is slightly more available at lower PH.... I just found this. See what ya think.
Nitrogen
The form of N and the fate of N in the soil-plant system is probably the major driver of changes in soil pH in agricultural systems.
Nitrogen can be added to soils in many forms, but the predominant forms of fertilizer N used are urea (CO(NH2)2), monoammonium phosphate (NH4H2PO4), diammonium phosphate ((NH4)2HPO4), ammonium nitrate (NH4NO3), calcium ammonium nitrate (CaCO3+NH4(NO3)) ammonium sulfate ((NH4)2SO4), urea ammonium nitrate (a mixture of urea and ammonium nitrate) and ammonium polyphosphate ([NH4PO3]n).
The key molecules of N in terms of changes in soil pH are the uncharged urea molecule ([CO(NH2)2]0), the cation ammonium (NH4+) and the anion nitrate (NO3-). The conversion of N from one form to the other involves the generation or consumption of acidity, , and the uptake of urea, ammonium or nitrate by plants will also affect acidity of soil (Figure 1).
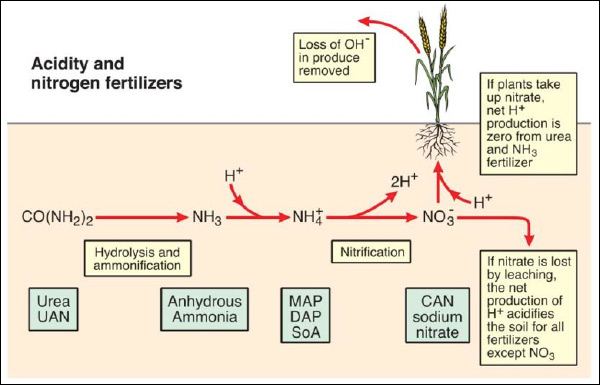
Figure 1. Soil acidity and nitrogen fertilizers (modified from (Davidson 1987)). MAP = monoammonium phosphate, DAP = diammonium phosphate, SoA = sulfate of ammonia, CAN = calcium ammonium nitrate, sodium nitrate
It can be seen in Figure 1 that ammonium-based fertilizers will acidify soil as they generate two H+ ions for each ammonium molecule nitrified to nitrate. The extent of acidification depends on whether the nitrate produced from ammonium is leached or is taken up by plants. If nitrate is taken up by plants the net acidification per molecule of ammonium is halved compared to the scenario when nitrate is leached. This is due to the consumption of one H+ ion (or excretion of OH-) for each molecule of nitrate taken up – this is often observed as pH increases in the rhizosphere (Smiley and Cook 1973). Anhydrous ammonia and urea have a lower acidification potential compared to ammonium-based products as one H+ ion is consumed in the conversion to ammonium. Nitrate-based fertilizers have no acidification potential and actually can increase soil pH as one H+ ion is absorbed by the plant (or OH- excreted) in the uptake of nitrate.
Phosphorus
The form of P fertilizer added to soil can affect soil acidity, principally through the release or gain of H+ ions by the phosphate molecule depending on soil pH (Figure 2). If phosphoric acid (PA) is added to soil, the molecule will always acidify soil as H+ ions will be released - one H+ ion if the soil pH is less than ~6.2 and two H+ ions is the soil pH is above 8.2. Monoammonium phosphate (MAP), single superphosphate (SSP) and triple superphosphate (TSP) all add P to soil in the form of the H2PO4- ion, which can acidify soil with a pH greater than 7.2 but has no effect on soil pH in acidic soils. The form of P in diammonium phosphate (DAP) is HPO42- which can make acidic soils (pH<7.2) more alkaline but has no effect on soil with a pH>7.2. The hydrolysis of ammonium polyphosphate (APP), where the P present as the P2O74- molecule converts to HPO42-, is pH neutral and hence any acidification due to adding P can be regarded as similar to DAP. SSP or TSP are sometimes declared to cause soil acidification due to reaction products being very acidic;
Ca(H2PO4)2+ 2H2O è CaHPO4 + H+ + H2PO4-
but in soils with pH values less than 7.7 the following reaction neutralizes the acidity produced so that there is no net acidification;
CaHPO4 + H2O è Ca2+ + H2PO4- + OH-
In high pH soils (pH >7.2), dissociation of H+ ion from the H2PO4- molecule will generate some acidity.
Crop uptake of P has little effect on soil acidity due to the small amounts of fertilizer P taken up in any one year – hence fertilizer chemistry dominates pH changes and significant differences in rhizosphere pH have not been observed for uptake of different orthophosphate ions.
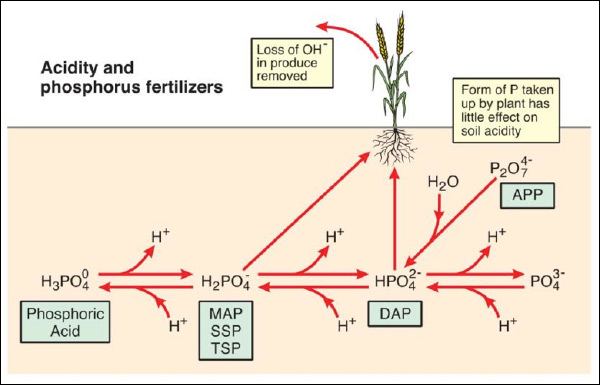
Figure 2. Soil acidity and P fertilizers. MAP = monoammonium phosphate, DAP = diammonium phosphate,
SSP = single superphosphate, TSP = triple superphosphate, APP = ammonium polyphosphate.
Sulfur
The form of S fertilizer added to soil can affect soil acidity, principally through the release of H+ ions by the addition of elemental S (S0) or thiosulfate (S2O32-, in ammonium thiosulfate - ATS) (Figure 3). However, the amounts of S added to soil and taken up by plants are generally small in comparison to N.
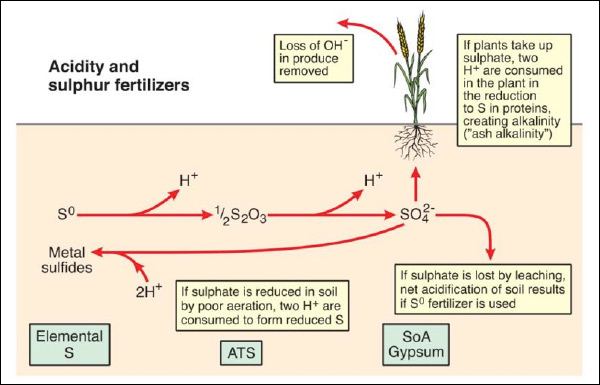
Figure 3. Soil acidity and S fertilizers. S0 = elemental S, ATS = ammonium thiosulfate, SoA = sulfate of ammonia.
For each molecule of S0 added to soil, two H+ ions will be generated, and these can be balanced through plant uptake by either uptake of H+ (same as excretion of OH- ions) or the generation of OH- (effectively organic anions) within the plant to form alkaline plant material (“ash alkalinity”). Where produce is removed (which is often the case in agricultural systems) net acidification of soil will occur if S0 or ATS are used.
Potassium
The form in which K is added to soil – either muriate of potash (KCl) or sulfate of potash (K2SO4) - has no effect on soil acidification.
Does that take into account this is hydro, not soil? And that these are all synthetic nutes (in a sterile resi) that are designed to work for this application?
Yeah, N is easier to uptake at lower pH but tbh it’s uptake occurs throughout the pH range. It’s the P & K that need higher pH’s later in the cycle.





