Frankster
Never trust a doctor who's plants have died.
Supporter
- 5,188
- 313
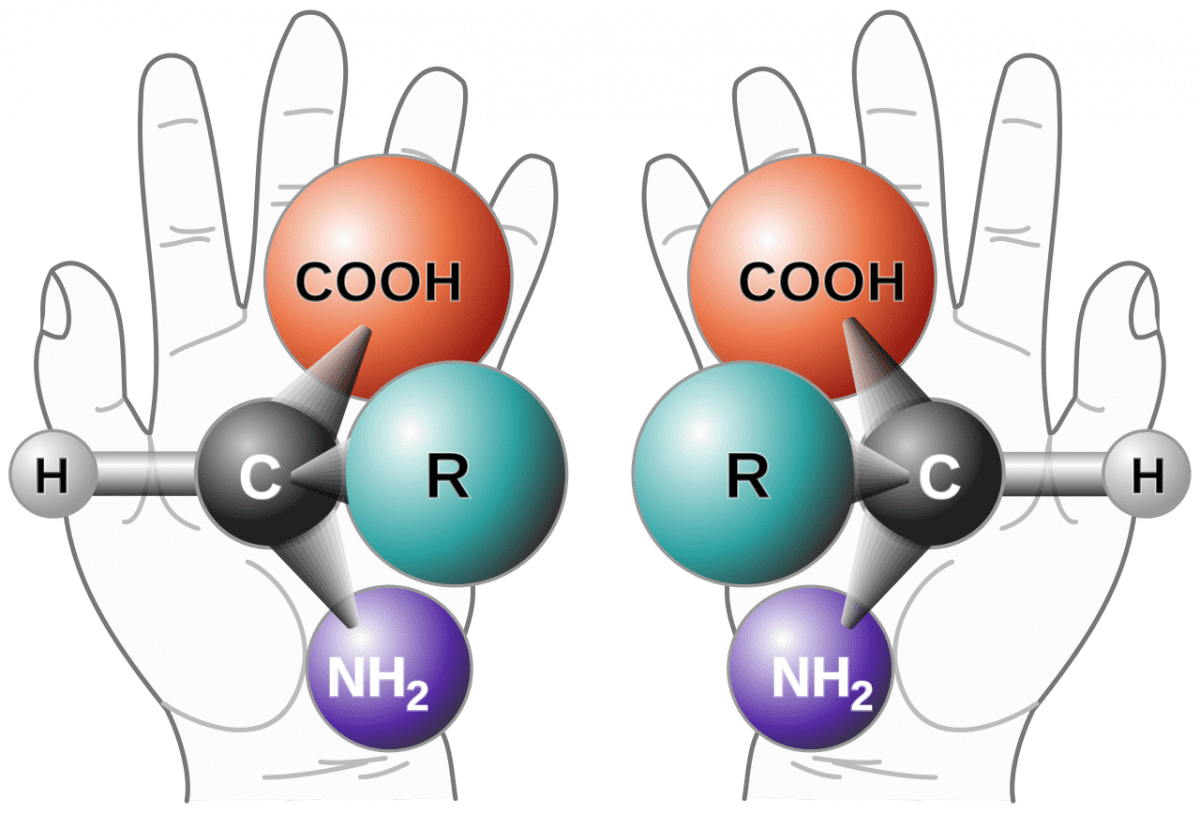
racemization is a conversion, by heat or by chemical reaction, of an optically active compound into a racemic (optically inactive) form. Half of the optically active substance becomes its mirror image (enantiomer) referred as racemic mixtures (i.e. contain equal amount of (+) and (−) forms). If the racemization results in a mixture where the D & L enantiomers are present in equal quantities, the resulting sample is described as a racemate. Racemization can proceed through a number of different mechanisms, and it has particular significance in pharmacology as different enantiomers may have different pharmaceutical effects.
Of note, the L form of amino acids and the D form of sugars (primarily glucose) are usually the biologically reactive form.
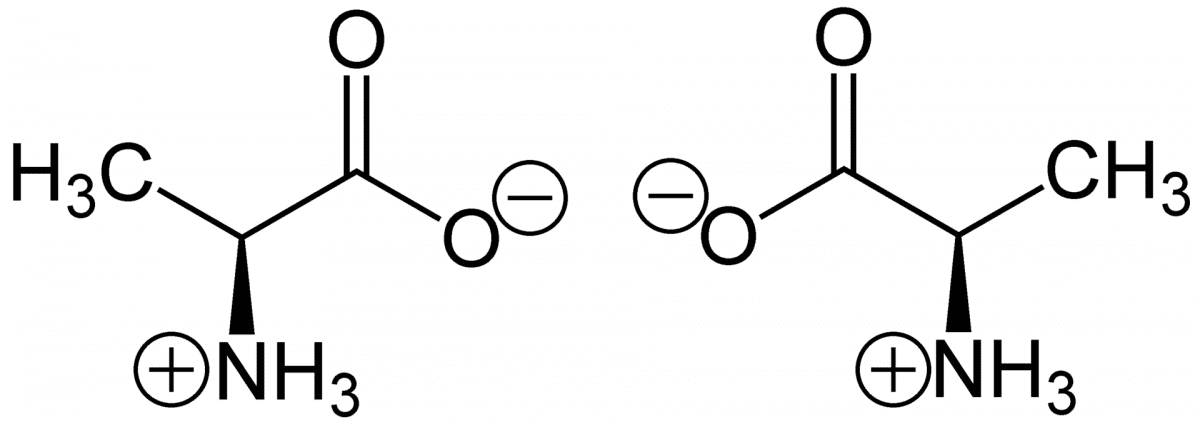
(S)-Alanine (left) and (R)-alanine (right) in zwitterionic form at neutral pH
The stereoselective nature of most biochemical reactions meant that different enantiomers of a chemical may have different properties and effects on a person. Many psychotropic drugs show differing activity or efficacy between isomers, e.g. amphetamine is often dispensed as racemic salts while the more active dextroamphetamine is reserved for refractory cases or more severe indications; another example is methadone, of which one isomer has activity as an opioid agonist and the other as an NMDA antagonist.
Racemization of drugs can occur in vivo. Thalidomide as the (R) enantiomer is effective against morning sickness, while the (S) enantiomer is teratogenic, causing birth defects. The configurational stability of a drug is therefore an area of interest in pharmaceutical research. The production and analysis of enantiomers in the pharmaceutical industry is studied in the field of chiral organic synthesis.
Of note, the L form of amino acids and the D form of sugars (primarily glucose) are usually the biologically reactive form.
(S)-Alanine (left) and (R)-alanine (right) in zwitterionic form at neutral pH
The stereoselective nature of most biochemical reactions meant that different enantiomers of a chemical may have different properties and effects on a person. Many psychotropic drugs show differing activity or efficacy between isomers, e.g. amphetamine is often dispensed as racemic salts while the more active dextroamphetamine is reserved for refractory cases or more severe indications; another example is methadone, of which one isomer has activity as an opioid agonist and the other as an NMDA antagonist.
Racemization of drugs can occur in vivo. Thalidomide as the (R) enantiomer is effective against morning sickness, while the (S) enantiomer is teratogenic, causing birth defects. The configurational stability of a drug is therefore an area of interest in pharmaceutical research. The production and analysis of enantiomers in the pharmaceutical industry is studied in the field of chiral organic synthesis.






