DrMcSkunkins
Dabbling in Oil
- 3,901
- 263
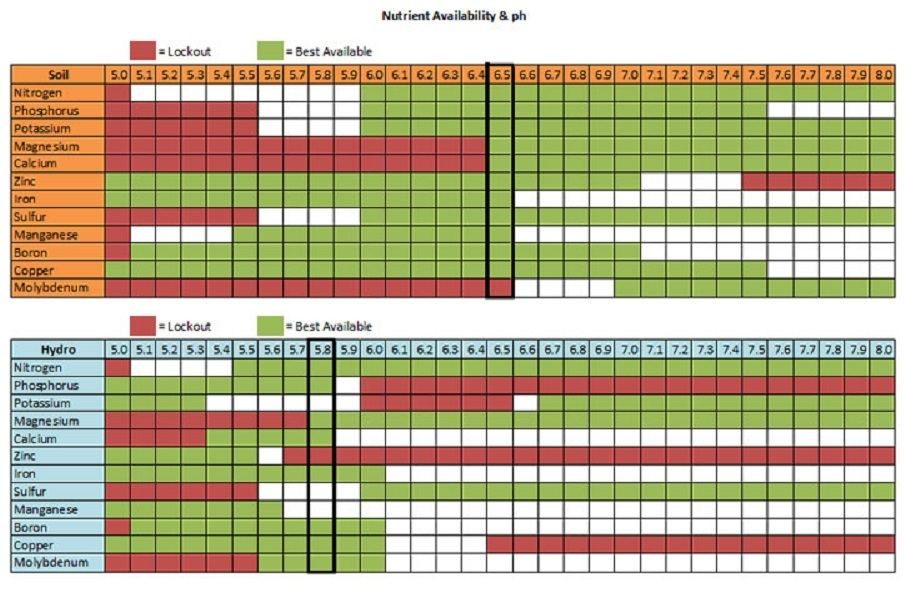
5.5 ph in hydro will lock out Mg,Mb, and S. I try to keep mine between 5.8 and 6.0

Ok, I've done perlite and I ended up dropping pH a wee bit lower than that, and a little tighter range--5.2-5.5. You might want to try adjusting pH before adding more fertilizer. I know what the charts say, and then I know what my plants said. I don't recall how many you have going, but maybe you can try raising for one or two, and dropping for one or two, see how they like it.5.5-6.0
Straight Perlite
My death stars and chemdogs seem to love it.
Thanks
Would you agree epsom salts wouldnt hurt?Nope, toxicities burn the plants. And since you say things got worse when you increased feeding, there's part of your tell. Also, you say pH'd. To what? What's your medium?
At this juncture, only if applied by foliar.Would you agree epsom salts wouldnt hurt?
I would agree it would hurt it moreWould you agree epsom salts wouldnt hurt?
I can see them starting to perk up a bit. I switched from GH calmag organic to the synthetic stuff. I've noticed the organic stuff creates a sludge in my reservoir. Also I had been using H&G Coco A&B. I just bought the aquaflakes A&B. Heard aquaflakes is more stable for nutrients that sit in a reservoir more than a few days. So we will see. Defiantly think me trying to keep the pH at 5.3-5.5 last week had an ill effect.
I would agree it would hurt it more
usually plants with phos issues in most cases will have root issues.
Improper pH level, irresponsible watering or over watering, soil that has too much moisture in it, and cold temperatures of lower than 60 degrees F. Temperature is especially a problem the the change is sudden
light intensity is another factor
It is less common for plants in a low-light environment to have a phosphorus deficiency since they perform photosynthesis at a lower rate. In contrast, plants in an intensely lit environment will be more likely to “use up” or go through the phosphorus that they have. This is simply because phosphorus is used in the process of photosynthesis. This means that when photosynthesis speeds up – which usually occurs when there is a large amount of light – then the rate of using phosphorus also increases.
Now being grown in Perlite and phos being mobile in plants which usually means it will show its ugly face on older leafs like in op's pictures
I like to ask if any new growth shows small new leafs ?? has plant slowed down in overall growth ?
The post above mentioning its only needed at the beginning and 1st week in flower.
I say your wrong it plays a key rollm in photosynthesis, respiration, energy storage and transfer, cell division, cell enlargement and several other processes.
P is needed for all of plants life this is why its on a essential in list NPK the main macro
Grow on dude soon you will have it masteredHoly fuck guys. Thank you all for the incredible support you have all provided.
