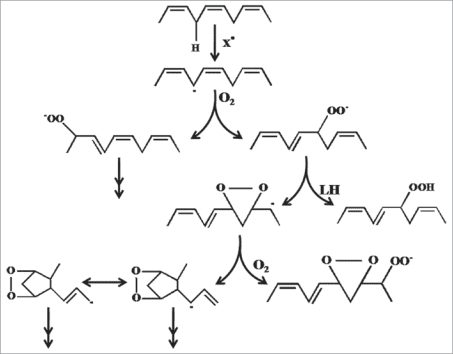
The reason UV is so bad, for all of the reasons it is bad, is because the energies of the radiation coincide very closely with many of the bond energies present in organic molecules. Because energy levels of electrons (which are responsible for bonding) are discrete, they require particles or energies of specific magnitudes which coincide with those discrete energy levels in order to "dislodge" the electron and thus the bond.
UV spectrum just happens to contain energy levels which coincide with a great deal of organic bonds. That includes EVERYTHING in your extract material.
I mean you can for real get some really funky shit happening in a mixture like that with UV. Do not recommend. UV is honestly 1 million percent more powerful than people have respect for. It's probably what has driven forward almost all of the relevant chemistry of this planet throughout history.
Adding UV is essentially no different than adding a reagent (and chemists treat it this way).
All good points, most of which I agree with, so may we discuss perspective?
All evidence that I've seen shows that UV definitely does cause the aromatic Alkene
terpenes, including the diterpene cannabinoids to decay, but we should also consider that the resins evolved in and exist in direct sunlight and sun screening is listed as one of their properties. That suggests that it isn't a rapid process in the absence of significant heat, differences in PH, or dielectric constants.
Adding EtOH is of course a variable, but as a simple Alkane alcohol, it is also pretty benign.
I believe that your points are all technically valid, but question the probable degree of exposure, overall perspective, and the level of concern that should be assigned to it. Have you seen any research on the subject, that might be enlightening enough to sway my flawed thinking?