Steve Gray
- 53
- 8
is is healthy to use this honey bee extractor ive been hearing about? i read tht its some kind of specially chemically resistant plastic but im not sure and i wanted to make sure. what do yall think about it
Have one...save the $....get better quality.is is healthy to use this honey bee extractor ive been hearing about? i read tht its some kind of specially chemically resistant plastic but im not sure and i wanted to make sure. what do yall think about it
I always read your Posts, Greywolf...always good info. thanksThe Honeybee extractor is made of Polypropylene, which is butane resistant. See http://www.coleparmer.com/Chemical-Resistance
The drawback that I see with the design, is that it is too large an ID for efficient single pass column extraction. Better to use an extractor under an inch ID and for a small unit, consider the stainless turkey baster from Bed, Breakfast, and Beyond for $7.99.
Tis true that a turkey baster would be a poor choice for processing pounds, and that and Iso bath would trump it.
The fastest and cheapest way that we've found to process pounds, is the Terpenator Mk III, which will process around 1.3 pounds per hour running full tilt, at a cost of about $0.038 (three point eight cents) per gram.
The Honeybee extractor is made of Polypropylene, which is butane resistant. See http://www.coleparmer.com/Chemical-Resistance
The drawback that I see with the design, is that it is too large an ID for efficient single pass column extraction. Better to use an extractor under an inch ID and for a small unit, consider the stainless turkey baster from Bed, Breakfast, and Beyond for $7.99.
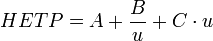
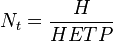
For this purpose a wider and shorter column (subject to optimization) would be better in terms of solvent required for complete column extraction.
tea and crumpets with graywolf...an extractors dream :DDespite the math, the results fly in the face of our practical experimentation. Our yield was higher from a 1" tube, than a 1 1/2" tube.
Removing the material and examining under a microscope revealed that the flow channeled and missed more trichomes heads in the larger diameter column, especially towards the injection end.
True story that soaking works better and is less sensitve to column ID. A thermos, Terpenator, or Lil Terp both get higher yield than a single pass column.
If you are in the area, by all means swing by for tea and crumpets!
Despite the math, the results fly in the face of our practical experimentation. Our yield was higher from a 1" tube, than a 1 1/2" tube.
Removing the material and examining under a microscope revealed that the flow channeled and missed more trichomes heads in the larger diameter column, especially towards the injection end.
True story that soaking works better and is less sensitve to column ID. A thermos, Terpenator, or Lil Terp both get higher yield than a single pass column.
If you are in the area, by all means swing by for tea and crumpets!
be careful what you ask for lol one hit of graywolf's oil and you'll be feeling it for about 7 hours lol Thanks Graywolf! lol definitely the tastiest and strongest oil i ever had the pleasure of trying loltea and crumpets with graywolf...an extractors dream :D
Considering the simplicity, accessibility and safety I give my vote for the thermos.True story that soaking works better and is less sensitve to column ID. A thermos, Terpenator, or Lil Terp both get higher yield than a single pass column.
Considering the simplicity, accessibility and safety I give my vote for the thermos.
Yes, the channeling is the issue to be sure--but this is caused by many factors (perhaps the least of which is the diameter of the column--although this does have an effect under these specific circumstances due to pressure concerns and instability of flow).
To my mind, the best methodology would be to combat the channeling by different methods (such as splitting I mentioned above, tighter packing, or--most importantly--modulation of particle size).
Other factors which are important here:
1. Flow rate.
2. Constancy of flow rate.
3. Solvent delivery apparatus: Is it one hole of a significantly smaller diameter than the column delivering the solvent? If so this is a huge problem--and the most likely culprit behind channeling. This is why, to my mind, hexane is the best choice for a solvent. For no reason other than it is a liquid. This type of extraction is meant for a solvent in the liquid phase--not one which is rapidly interconverting between phases during its pass through the column. This is all but guaranteed to cause channeling. For lack of a better way to say it, it's just not the correct way to do this--that is why your terpenator is so badass, it's allowing proper contact with the material. In a sentence, using butane doesn't allow fine control over #2 at atmospheric pressure, and that is going to cause channeling (and the terpenator is a soak correct--so no channeling there).
Discussing which is the best method by which to extract with butane at atmospheric pressure is really counter-intuitive. The answer is that the best method is not to do this at all. There is almost no opportunity to control either flow rate or injection geometry (I can't think of a better way to say that--I'm sure I need an engineering word that I don't know).
The addition of sand in "splitting" helps by reducing channeling through uniform particle size, and this ensures that as solvent leaves the sand layer--it will do so along the entire area of the column (i.e. flow being directed which matches the diameter of the column--rather than a single hole).
A good practice would be to pack the top of a column with sand or silica anyway to achieve this effect at the initial injection point.
I realize that isn't going to stop anyone--but I think if we're going to tackle these issues that we, as a community, should understand them.
In short, it's not failing to work necessarily because of the diameter of the tube--although if we restrict ourselves in this area we can get "better" results. However, better isn't best--and in this case it can't even be classified as proper.
Injection end always gets fresh solvent, and output end always gets saturated.
This is why knowing about what you're extracting and your expected yields as well as relevant solubility constants if you want to do this as an industrial process.
You can avoid efficiency loss by modulating column length or adjusting flow rate--but obviously that isn't happening with butanes because its going into gas phase and such with a blast-through technique.
