Aqua Man
- 26,480
- 638
They are talking completely sterile… add root and thats not sterile. Here shows just water supply and H2o2 last for 5.5 and just over 1 hr.
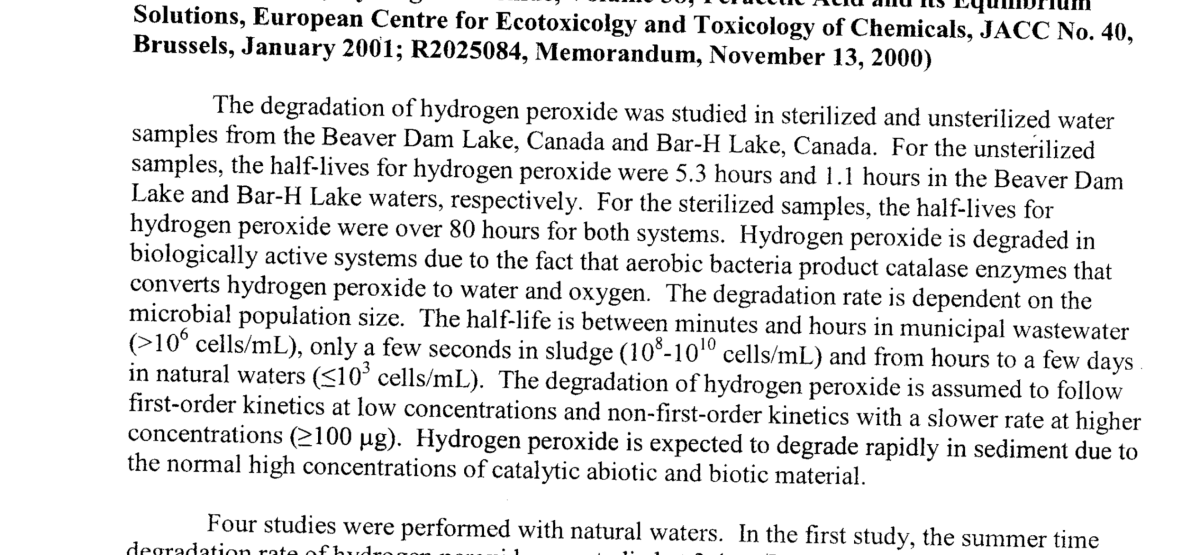
I've always scrutinized that in my head, coming from doing some soft core microbiology. IIRC, you'll find certain bacteria even in bottles isopropyl and h2o2. Surely once you open them in air not under a flow hood.no you are confusing sterile with a name we use… they are talking sterile like medical
100% agreed, how long roughly does it take you to achieve this? I was aiming for that but had to transplant a little earlier than I liked due to temp issues with my seedling raiser and I had to go to work for a period of time, I had two nodes on most, some have a long single tap root and some have much shorter but much more roots out of the bottom of the basket. 3 weeks my bay burgers spent before transplanting.8-12" roots and 3+ nodes is safe transplant. Less than that yoi run the risk of shock and death
Exposure and concentration are far different in our systems then in a bottleI've always scrutinized that in my head, coming from doing some soft core microbiology. IIRC, you'll find certain bacteria even in bottles isopropyl and h2o2. Surely once you open them in air not under a flow hood.
What we run are sanitized systems.
I'm using strips that supposedly measure the PPM of H2O2, not dissolved solids.Ppm is not necessarily completely h2o2 but simply a measure of dissolved solids
Ahhh i see. Your results are probably correct. The dose and concentration are going to be the key.I'm using strips that supposedly measure the PPM of H2O2, not dissolved solids.
I can only tell you what I actually see in real life in these systems, beyond that you decide.
Either my strips are not truly measuring H2O2 concentrations, or it lasts much longer than people think.
also can I say this as a noob without being roasted, but aren't these plants way too small for cal and mag supplementation? the plants requirements are quite minimal at this stage, are they really absorbing that much that it needs to be supplemented? Also using H&G I don't think so. I'd biff it till you need it. You don't need anything but the essentials in the res, the rest will just do harm in the early stages IMO
hey mate decided to share a pic with you.
so my last grow died from root rot, last grow was first NFT grow, im now onto second, still learning, its not smooth sailing but im better at "fixing" things this time, this crop still might die, im not counting my chickens yet, but this is a 100% legit time line. 15/9 start germ, 19/9 transplanted to Rockwool 3/10 day 1 Veg. Today is the start of week 2 veg, this plant had a single 16cm tap root on it last Monday. All I have in my soup is aqua flakes A&B in minimal amounts and super thrive (get yourself some, the best). Ive been top watering morning and night, they seem to like it. EC 0.5 (0.7 inc tap) up until they went into NFT system they were just given top watering with a drop of super thrive and a drop of roots excel per 1L, anything else was too much.
View attachment 1290733
im really happy dude!!
Ahhh i see. Your results are probably correct. The dose and concentration are going to be the key.
Just so i can understand how are you testing it and do you notice any difference in them over the grow?
like initially ot lasts days and when the roots have become very established you need to add more or more frequently?
what dose are you using of what %?
There is a reason your seeing those results… just cant say what they are
Ahhh ok that makes complete sense… again i get caught thinking linearly.You probably remember when I recently started up my mothballed system and it was really nasty due to undrained lines and chiller.
I filled it with RO and started all the pumps.
I then put in a lot of 12% (maybe .5L? didn't measure) and then ran the system that way for a day. I used the water to clean all parts of the system and then drained it. It was over 100PPM, which is the max for these strips.
After I drained and added new RO day 2, there was still sufficient H2O2 in the water to read 100PPM. I let it go another day.
Drained and added RO and the H2O2 is now measurable. In the 50PPM range. I let it run for a week that way, and it only dropped slightly.
This is why I say it reacts more slowly in a situation where the bio load is low. Of course it is not sterile, I just meant that all significant life was gone.
In order to get to zero, I had to sully drain once more, and even vacuum out the remaining crannies with a shop vac, then add RO back in.
I can bench test this and show this phenomenon quite easily. But I am certain that H2O2 reacts differently depending on the load, if these strips are to be believed.
In terms of running it while growing, yes, any organic matter, including roots, will consume H2O2 more quickly. I have seen this too.
I have not run a sterile system in a long time, so I do not have any additional documentation to share on that.
You probably remember when I recently started up my mothballed system and it was really nasty due to undrained lines and chiller.
I filled it with RO and started all the pumps.
I then put in a lot of 12% (maybe .5L? didn't measure) and then ran the system that way for a day. I used the water to clean all parts of the system and then drained it. It was over 100PPM, which is the max for these strips.
After I drained and added new RO day 2, there was still sufficient H2O2 in the water to read 100PPM. I let it go another day.
Drained and added RO and the H2O2 is now measurable. In the 50PPM range. I let it run for a week that way, and it only dropped slightly.
This is why I say it reacts more slowly in a situation where the bio load is low. Of course it is not sterile, I just meant that all significant life was gone.
In order to get to zero, I had to sully drain once more, and even vacuum out the remaining crannies with a shop vac, then add RO back in.
I can bench test this and show this phenomenon quite easily. But I am certain that H2O2 reacts differently depending on the load, if these strips are to be believed.
In terms of running it while growing, yes, any organic matter, including roots, will consume H2O2 more quickly. I have seen this too.
I have not run a sterile system in a long time, so I do not have any additional documentation to share on that.
First day of veg is the day they went into the system for me as that’s kind of what I’ve seen as the standard around.Yeah, this is what I like to see - people actually doing legit testing instead of just guessing. IMO there are way too many variables in everyone's environment and grow to do a magic 'one size fits all' for things like this. I see so much of the guessing game thrown around on the net, and the thing is, just because someone got through a grow with what they were doing, without actually testing to see where their levels of free chlorine, h2o2, 'whatever have you', etc are at - they have no real way of knowing.
Maybe .... their chlorine/h2o2/etc off gassed and they just didn't have anything in their water/air/environment to begin with that would cause them problems. They would automatically assume "I added this much of X and I had no problems!". That's how scientific evidence is formulated for anything - you have a hypothesis, you actually test for it and pull back some hard data, and it either confirms or busts the hypothesis.
There's so many people who just blindly grow, following random conflicting data online without ever doing any real testing/research on their own that it makes it hard for, at least someone like myself who is more of an evidence based observer/learner, hard to justify/believe. I know to a lot of people - especially those who have zero/minimal problems - it seems stupid, counterproductive, or a waste of time, but ... imo, if someone is really trying to sit down and truly find underlying issues and really sit down and gain an in-depth understanding of what is actually going on, you're only going to get there by actually doing actual, real testing to confirm or deny your assumptions.
I personally can see this level of actual testing/data gathering as beneficial. I see absolutely no downsides to it whatsoever. Some will argue that growing is an art form, others will argue it's a science. Can't please everyone.
Personally, I have found the old 'don't give seedlings ANYTHING until 3 weeks/3 nodes' advice I've seen thrown around the internet countless times to be utter nonsense. I believe it stems from old growers, who came from hot soil. In Hyro, from what I've seen and experimented with - this deprives the plants and leaves the deficient of nutrients.
I also think this is *very* dependent upon the water you're using and what's already in it. I think a lot of people who still go by this probably have really poor water that's very high PPM - and that allows them to get away with not giving any nutes. This last attempt of mine when I actually started mine off with about 220tds (a mix of calmag, Aquaflakes A and B) - they were the greenest, best looking seedlings I've *EVER* had. Again, I'm a noob, and obviously am having issues, but ... this is what my testing has shown me after a LOT of starting over to all this root rot over the lat year+.
Looking fantastic! I appreciate your very precise timeline and dates of when you started, vs transplanted, vs considered it in veg.
I've never really understood exactly when the 'seedling' stage ends, the the 'veg' stage begins. I've seen answers all over the board related to that.
What are numbers but abstractions of realities?
It'd do good to move your mindset from thinking people "blindly" do "random" things that reap consistent results.
We can measure and replicate procedures without spreadsheets and data logs. We can identify erroneous steps or constituents without chemical analyses. And we can show others how to do it, too.
Over 15 years, I've used a myriad of different techniques with different processes with different components and only recently tracked even my pH. You find a way by trial and error - by building a relationship with the plant. And listening to others who have a better relationship with it - what you should be doing, regardless of whether they can provide you the data you believe you require.
If you think your water is bad, use different water.
Some people have an innate talent for horticulture. Others, including myself, could kill a cactus. I had to learn my green thumb with the internet and books as teachers.What are numbers but abstractions of realities?
It'd do good to move your mindset from thinking people "blindly" do "random" things that reap consistent results.
We can measure and replicate procedures without spreadsheets and data logs. We can identify erroneous steps or constituents without chemical analyses. And we can show others how to do it, too.
Over 15 years, I've used a myriad of different techniques with different processes with different components and only recently tracked even my pH. You find a way by trial and error - by building a relationship with the plant. And listening to others who have a better relationship with it - what you should be doing, regardless of whether they can provide you the data you believe you require.
If you think your water is bad, use different water.
Hey, let me stand up for Beluga here, I think you may be reading him wrong. He's genuinely trying to help you.You do you man. Like I've told and praised you for already - you clearly have a very good undestanding of growing, and know institutionally by now what/how to do things in a way that works for you.
Every person in almost every single field - growing or otherwise - who truly excels and has a massive amount of low level knowledge of how how things work has gotten there from test based research. Knowledge is never a bad thing. We as a species wouldn't even be where we are today, without countless minds and individuals doing scientific research/testing.
I really think it's actually kind of funny that you're basically mocking me for wanting to actually test and gather legitimate data on various aspects of things. Clearly you're intelligent, but that's how we evolve and learn as a species. Look at our own understanding of our reality. Look at the medical field. It's all done through test based research. How can gathering data and doing tests ever be a bad thing?
Anyway - I'm not going to argue about it with you any more. Clearly you're a very knowledgeable, experienced, and capable grower. I commend you for your experience and expertise, however.... I don't think you're doing anyone any favors by making fun of them for wanting to actually gather data on various aspects of things and gain a better, more in-depth understanding of what's truly going on at a fundamental level instead of just assuming/guessing.
Thanks, its a hobby. I do it for the lols.I love your test based methodology. Absolutely fantastic!
Some people have an innate talent for horticulture. Others, including myself, could kill a cactus. I had to learn my green thumb with the internet and books as teachers.
Ever hear of the 7 intelligences?
View attachment 1290757
I'm ohhh so purple. Somehow you straddle blue and purple, and to me that is unusual. Most natural born growers tend to be more "creative" types in addition to naturalistic. And on top of that you are a fish.
