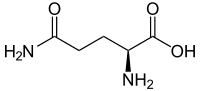
PGA stands for poly-glutamic acid. Glutamine is an amino acid with an amine (specifically an amide) side chain, seen here:
The left side of this molecule (beyond the first NH2 from the right) is the side chain.
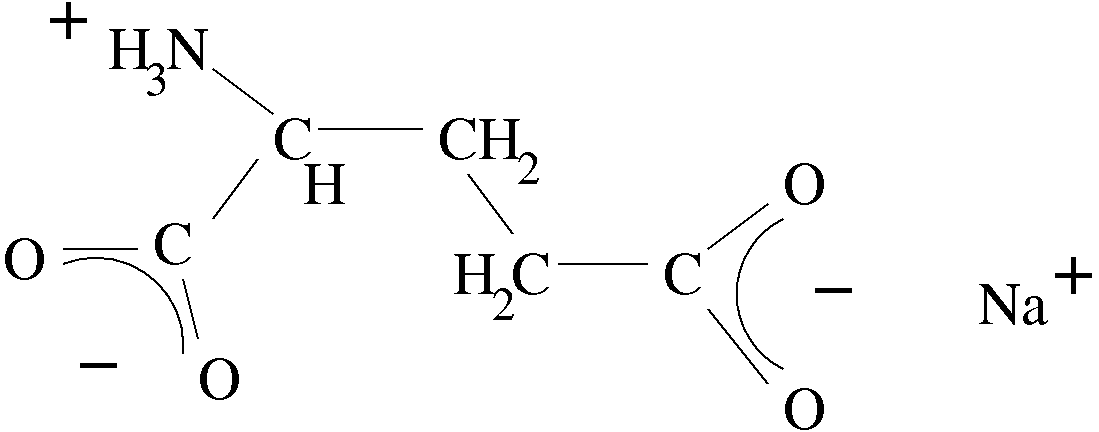
In glutamate this side chain is modified. Glutamate is a precursor to glutamine typically in biosynthesis. The side chain is modified thusly (ignore Na):
A hydroxyl group has replaced the amino group in glutamine. This makes the side chain acidic rather than basic. This hydroxyl groups on both ends are actually deprotonated at biological pH which means the carboxylic acid (protonated form) into a carboxylate (deprotonated form), seen here (again ignore the Na ion, it's simply there to "balance" the molecule ionically--also note the orientation is flipped left to right in this picture):
^^That thing is glutamate, sodium gluatamate to be specific.
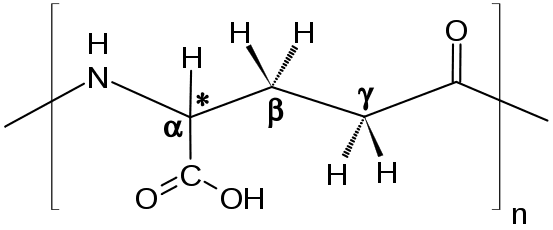
The "y" in yPGA is actually a "gamma". This refers to a type of linkage wherein the amino group forms (leftmost in the above picture) a peptide bond (amide bond) with the carboxyl group on the side chain of a second glutamate (rightmost in the above picture).
This looks something like what's seen on wiki:
The "n" here as well as the trailing bonds denote that this bond can be made many times. The "poly" refers to the fact that many such bonds are made in yPGA. This thing is essentially a big amino acid chain, really big.
This is of particular interest to us because water soluble polyorganic acids of greater size than 1250-1500 Da (daltons) increase fertilizer uptake in plants and therefore promote plant growth. To use a term we're all familiar with by now, these big polyorganic acids help to chelate fertilizers.
So, in short yPGA is good. More is better.